Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system
- PMID: 24420448
- PMCID: PMC3928514
- DOI: 10.1007/s13365-013-0227-1
Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system
Erratum in
-
Correction to: Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system.J Neurovirol. 2025 Jun;31(3):302-303. doi: 10.1007/s13365-025-01266-7. J Neurovirol. 2025. PMID: 40536660 No abstract available.
Abstract
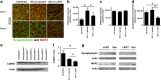
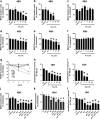
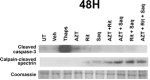
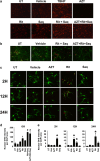
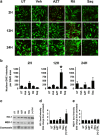
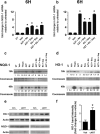
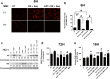
HIV-associated neurocognitive disorder (HAND), characterized by a wide spectrum of behavioral, cognitive, and motor dysfunctions, continues to affect approximately 50 % of HIV(+) patients despite the success of combination antiretroviral drug therapy (cART) in the periphery. Of note, potential toxicity of antiretroviral drugs in the central nervous system (CNS) remains remarkably underexplored and may contribute to the persistence of HAND in the cART era. Previous studies have shown antiretrovirals (ARVs) to be neurotoxic in the peripheral nervous system in vivo and in peripheral neurons in vitro. Alterations in lipid and protein metabolism, mitochondrial damage, and oxidative stress all play a role in peripheral ARV neurotoxicity. We hypothesized that ARVs also induce cellular stresses in the CNS, ultimately leading to neuronal damage and contributing to the changing clinical and pathological picture seen in HIV-positive patients in the cART era. In this report, we show that ARVs are neurotoxic in the CNS in both pigtail macaques and rats in vivo. Furthermore, in vitro, ARVs lead to accumulation of reactive oxygen species (ROS), and ultimately induction of neuronal damage and death. Whereas ARVs alone caused some activation of the endogenous antioxidant response in vitro, augmentation of this response by a fumaric acid ester, monomethyl fumarate (MMF), blocked ARV-induced ROS generation, and neuronal damage/death. These findings implicate oxidative stress as a contributor to the underlying mechanisms of ARV-induced neurotoxicity and will provide an access point for adjunctive therapies to complement ARV therapy and reduce neurotoxicity in this patient population.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
References
-
- Anonymous (2011) Trial watch: phase III success for Biogen’s oral multiple sclerosis therapy. Nature Reviews Drug Discovery 10:404 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS056885/NS/NINDS NIH HHS/United States
- T32 NS007180/NS/NINDS NIH HHS/United States
- AG000255/AG/NIA NIH HHS/United States
- R01 DA022339/DA/NIDA NIH HHS/United States
- MH070306/MH/NIMH NIH HHS/United States
- P01 MH070306/MH/NIMH NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- NS27405/NS/NINDS NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- NS043994/NS/NINDS NIH HHS/United States
- DA22339/DA/NIDA NIH HHS/United States
- MH083517/MH/NIMH NIH HHS/United States
- K02 DA018678/DA/NIDA NIH HHS/United States
- T32 GM007517/GM/NIGMS NIH HHS/United States
- CA133470/CA/NCI NIH HHS/United States
- R01 NS043994/NS/NINDS NIH HHS/United States
- R01 MH098742/MH/NIMH NIH HHS/United States
- DA18678/DA/NIDA NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- T32 GM008076/GM/NIGMS NIH HHS/United States
- R01 MH095671/MH/NIMH NIH HHS/United States
- R01 MH083517/MH/NIMH NIH HHS/United States
- R01 CA133470/CA/NCI NIH HHS/United States
- P01 NS027405/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

