Cardiac hypoxia imaging: second-generation analogues of 64Cu-ATSM
- PMID: 24421288
- PMCID: PMC6214505
- DOI: 10.2967/jnumed.113.129015
Cardiac hypoxia imaging: second-generation analogues of 64Cu-ATSM
Abstract
Myocardial hypoxia is an attractive target for diagnostic and prognostic imaging, but current approaches are insufficiently sensitive for clinical use. The PET tracer copper(II)-diacetyl-bis(N4-methylthiosemicarbazone) ((64)Cu-ATSM) has promise, but its selectivity and sensitivity could be improved by structural modification. We have therefore evaluated a range of (64)Cu-ATSM analogs for imaging hypoxic myocardium.
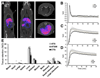
Methods: Isolated rat hearts (n = 5/group) were perfused with normoxic buffer for 30 min and then hypoxic buffer for 45 min within a custom-built triple-γ-detector system to quantify radiotracer infusion, hypoxia-dependent cardiac uptake, and washout. A 1-MBq bolus of each candidate tracer (and (18)F-fluoromisonidazole for comparative purposes) was injected into the arterial line during normoxia, and during early and late hypoxia, and their hypoxia selectivity and pharmacokinetics were evaluated. The in vivo pharmacokinetics of promising candidates in healthy rats were then assessed by PET imaging and biodistribution.
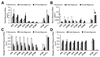
Results: All tested analogs exhibited hypoxia sensitivity within 5 min. Complexes less lipophilic than (64)Cu-ATSM provided significant gains in hypoxic-to-normoxic contrast (14:1 for (64)Cu-2,3-butanedione bis(thiosemicarbazone) (ATS), 17:1 for (64)Cu-2,3-pentanedione bis(thiosemicarbazone) (CTS), 8:1 for (64)Cu-ATSM, P < 0.05). Hypoxic first-pass uptake was 78.2% ± 7.2% for (64)Cu-ATS and 70.7% ± 14.5% for (64)Cu-CTS, compared with 63.9% ± 11.7% for (64)Cu-ATSM. Cardiac retention of (18)F-fluoromisonidazole increased from 0.44% ± 0.17% during normoxia to 2.24% ± 0.08% during hypoxia. In vivo, normoxic cardiac retention of (64)Cu-CTS was significantly lower than that of (64)Cu-ATSM and (64)Cu-ATS (0.13% ± 0.02% vs. 0.25% ± 0.04% and 0.24% ± 0.03% injected dose, P < 0.05), with retention of all 3 tracers falling to less than 0.7% injected dose within 6 min. (64)Cu-CTS also exhibited lower uptake in liver and lung.
Conclusion: (64)Cu-ATS and (64)Cu-CTS exhibit better cardiac hypoxia selectivity and imaging characteristics than the current lead hypoxia tracers, (64)Cu-ATSM and (18)F-fluoromisonidazole.
Keywords: 18FMISO; 64Cu-ATSM; PET; bis(thiosemicarbazones); cardiac ischemia; hypoxia.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


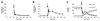

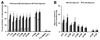
Similar articles
-
64Cu-CTS: A Promising Radiopharmaceutical for the Identification of Low-Grade Cardiac Hypoxia by PET.J Nucl Med. 2015 Jun;56(6):921-6. doi: 10.2967/jnumed.114.148353. Epub 2015 Apr 16. J Nucl Med. 2015. PMID: 25883129
-
Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone).J Nucl Med. 2006 Jun;47(6):989-98. J Nucl Med. 2006. PMID: 16741309
-
Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models.Int J Radiat Oncol Biol Phys. 2005 Apr 1;61(5):1493-502. doi: 10.1016/j.ijrobp.2004.12.057. Int J Radiat Oncol Biol Phys. 2005. PMID: 15817355
-
Hypoxia imaging and theranostic potential of [64Cu][Cu(ATSM)] and ionic Cu(II) salts: a review of current evidence and discussion of the retention mechanisms.EJNMMI Res. 2020 Apr 9;10(1):33. doi: 10.1186/s13550-020-00621-5. EJNMMI Res. 2020. PMID: 32274601 Free PMC article. Review.
-
Contribution of [64Cu]-ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F]-MISO--a selected review.Nucl Med Rev Cent East Eur. 2011;14(2):90-5. doi: 10.5603/nmr.2011.00022. Nucl Med Rev Cent East Eur. 2011. PMID: 22219149 Review.
Cited by
-
Creation of clinically relevant model of chronic heart failure: Application of multi-modality imaging to define physiology.J Nucl Cardiol. 2015 Aug;22(4):673-6. doi: 10.1007/s12350-015-0081-z. Epub 2015 Feb 20. J Nucl Cardiol. 2015. PMID: 25698482 No abstract available.
-
Evaluation of hypoxia with copper-labeled diacetyl-bis(N-methylthiosemicarbazone).Semin Nucl Med. 2015 Mar;45(2):177-85. doi: 10.1053/j.semnuclmed.2014.10.003. Semin Nucl Med. 2015. PMID: 25704389 Free PMC article. Review.
-
2020 FDA TIDES (Peptides and Oligonucleotides) Harvest.Pharmaceuticals (Basel). 2021 Feb 11;14(2):145. doi: 10.3390/ph14020145. Pharmaceuticals (Basel). 2021. PMID: 33670364 Free PMC article. Review.
-
Molecular imaging of hypoxia in non-small-cell lung cancer.Eur J Nucl Med Mol Imaging. 2015 May;42(6):956-76. doi: 10.1007/s00259-015-3009-6. Epub 2015 Feb 21. Eur J Nucl Med Mol Imaging. 2015. PMID: 25701238 Review.
-
Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging.Molecules. 2017 Apr 17;22(4):641. doi: 10.3390/molecules22040641. Molecules. 2017. PMID: 28420176 Free PMC article.
References
-
- Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev. 2000;5:131–138. - PubMed
-
- Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. - PubMed
-
- Sinusas AJ. The potential of myocardial imaging with hypoxia markers. Semin Nucl Med. 1999;29:330–338. - PubMed
-
- Dearling JLJ, Lewis JS, Mullen GED, Rae MT, Zweit J, Blower PJ. Design of hypoxia-targeting radiopharmaceuticals: selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur J Nucl Med. 1998;25:788–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
