The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages
- PMID: 24421327
- PMCID: PMC3999755
- DOI: 10.1182/blood-2013-07-516948
The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages
Abstract
PSTPIP1 is a cytoskeletal adaptor and F-BAR protein that has been implicated in autoinflammatory disease, most notably in the PAPA syndrome: pyogenic sterile arthritis, pyoderma gangrenosum, and acne. However, the mechanism by which PSTPIP1 regulates the actin cytoskeleton and contributes to disease pathogenesis remains elusive. Here, we show that endogenous PSTPIP1 negatively regulates macrophage podosome organization and matrix degradation. We identify a novel PSTPIP1-R405C mutation in a patient presenting with aggressive pyoderma gangrenosum. Identification of this mutation reveals that PSTPIP1 regulates the balance of podosomes and filopodia in macrophages. The PSTPIP1-R405C mutation is in the SRC homology 3 (SH3) domain and impairs Wiskott-Aldrich syndrome protein (WASP) binding, but it does not affect interaction with protein-tyrosine phosphatase (PTP)-PEST. Accordingly, WASP inhibition reverses the elevated F-actin content, filopodia formation, and matrix degradation induced by PSTPIP1-R405C. Our results uncover a novel role for PSTPIP1 and WASP in orchestrating different types of actin-based protrusions. Our findings implicate the cytoskeletal regulatory functions of PSTPIP1 in the pathogenesis of pyoderma gangrenosum and suggest that the cytoskeleton is a rational target for therapeutic intervention in autoinflammatory disease.
Figures

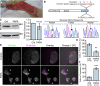
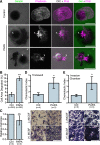
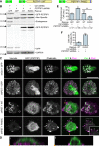

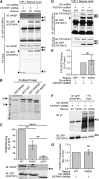

Comment in
-
Podosomes are disrupted in PAPA syndrome.Blood. 2014 Apr 24;123(17):2597-9. doi: 10.1182/blood-2014-01-551192. Blood. 2014. PMID: 24764557 No abstract available.
References
-
- Badolato R. Defects of leukocyte migration in primary immunodeficiencies. Eur J Immunol. 2013;43(6):1436–1440. - PubMed
-
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. - PubMed
-
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9(9):988–998. - PubMed
-
- Lokuta MA, Cooper KM, Aksentijevich I, Kastner DL, Huttenlocher A. Neutrophil chemotaxis in a patient with neonatal-onset multisystem inflammatory disease and Muckle-Wells syndrome. Ann Allergy Asthma Immunol. 2005;95(4):394–399. - PubMed
-
- Heath RJW, Insall RH. F-BAR domains: multifunctional regulators of membrane curvature. J Cell Sci. 2008;121(Pt 12):1951–1954. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- T32 GM008692/GM/NIGMS NIH HHS/United States
- T32 AI007635/AI/NIAID NIH HHS/United States
- GM008692/GM/NIGMS NIH HHS/United States
- HL-102926/HL/NHLBI NIH HHS/United States
- R01 AR059703/AR/NIAMS NIH HHS/United States
- HL-102925/HL/NHLBI NIH HHS/United States
- T32 HL007899/HL/NHLBI NIH HHS/United States
- HL-102923/HL/NHLBI NIH HHS/United States
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102926/HL/NHLBI NIH HHS/United States
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- HL-103010/HL/NHLBI NIH HHS/United States
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- F30 HL114143/HL/NHLBI NIH HHS/United States
- R01 CA085862/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- HL007899/HL/NHLBI NIH HHS/United States
- HL-102924/HL/NHLBI NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- CA085862/CA/NCI NIH HHS/United States
- UC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102924/HL/NHLBI NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- AR059703/AR/NIAMS NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- UC2 HL102925/HL/NHLBI NIH HHS/United States
- HL114143/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

