Motor-driven marginal band coiling promotes cell shape change during platelet activation
- PMID: 24421335
- PMCID: PMC3897189
- DOI: 10.1083/jcb.201306085
Motor-driven marginal band coiling promotes cell shape change during platelet activation
Abstract
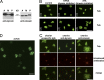
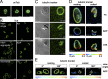
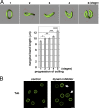
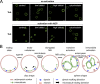
Platelets float in the blood as discoid particles. Their shape is maintained by microtubules organized in a ring structure, the so-called marginal band (MB), in the periphery of resting platelets. Platelets are activated after vessel injury and undergo a major shape change known as disc to sphere transition. It has been suggested that actomyosin tension induces the contraction of the MB to a smaller ring. In this paper, we show that antagonistic microtubule motors keep the MB in its resting state. During platelet activation, dynein slides microtubules apart, leading to MB extension rather than contraction. The MB then starts to coil, thereby inducing the spherical shape of activating platelets. Newly polymerizing microtubules within the coiled MB will then take a new path to form the smaller microtubule ring, in concerted action with actomyosin tension. These results present a new view of the platelet activation mechanism and reveal principal mechanistic features underlying cellular shape changes.
Figures

References
-
- Abramoff M.D., Magalhaes P.J., Ram S.J. 2004. Image Processing with ImageJ. Biophotonics International. 11:36–42
-
- Aslan J.E., Phillips K.G., Healy L.D., Itakura A., Pang J., McCarty O.J. 2013. Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation. Am. J. Physiol. Cell Physiol. 305:C1230–C1239 10.1152/ajpcell.00053.2013 - DOI - PMC - PubMed
-
- Behnke O., Forer A. 1998. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur. J. Haematol. Suppl. 61:3–23 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

