Reflex control of inflammation by sympathetic nerves, not the vagus
- PMID: 24421357
- PMCID: PMC3979618
- DOI: 10.1113/jphysiol.2013.268573
Reflex control of inflammation by sympathetic nerves, not the vagus
Abstract
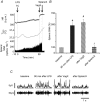
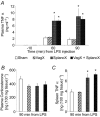
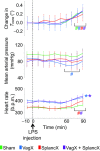
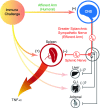
We investigated a neural reflex that controls the strength of inflammatory responses to immune challenge - the inflammatory reflex. In anaesthetized rats challenged with intravenous lipopolysaccharide (LPS, 60 μg kg(-1)), we found strong increases in plasma levels of the key inflammatory mediator tumour necrosis factor α (TNFα) 90 min later. Those levels were unaffected by previous bilateral cervical vagotomy, but were enhanced approximately 5-fold if the greater splanchnic sympathetic nerves had been cut. Sham surgery had no effect, and plasma corticosterone levels were unaffected by nerve sections, so could not explain this result. Electrophysiological recordings demonstrated that efferent neural activity in the splanchnic nerve and its splenic branch was strongly increased by LPS treatment. Splenic nerve activity was dependent on inputs from the splanchnic nerves: vagotomy had no effect on the activity in either nerve. Together, these data demonstrate that immune challenge with this dose of LPS activates a neural reflex that is powerful enough to cause an 80% suppression of the acute systemic inflammatory response. The efferent arm of this reflex is in the splanchnic sympathetic nerves, not the vagi as previously proposed. As with other physiological responses to immune challenge, the afferent pathway is presumptively humoral: the present data show that vagal afferents play no measurable part. Because inflammation sits at the gateway to immune responses, this reflex could play an important role in immune function as well as inflammatory diseases.
Figures


Comment in
-
Neural control of inflammation by the greater splanchnic nerves.Temperature (Austin). 2014 May 7;1(1):14-5. doi: 10.4161/temp.29135. eCollection 2014 Apr-Jun. Temperature (Austin). 2014. PMID: 27580886 Free PMC article.
References
-
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. - PubMed
-
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. - PubMed
-
- Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

