Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors
- PMID: 24421639
- PMCID: PMC3888267
- DOI: 10.2147/IJN.S55118
Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors
Abstract
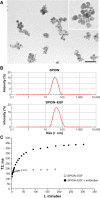
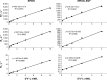
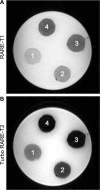
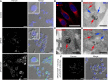
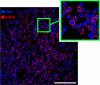
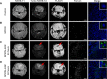
Superparamagnetic iron oxide nanoparticles (SPIONs) conjugated with recombinant human epidermal growth factor (SPION-EGF) were studied as a potential agent for magnetic resonance imaging contrast enhancement of malignant brain tumors. Synthesized conjugates were characterized by transmission electron microscopy, dynamic light scattering, and nuclear magnetic resonance relaxometry. The interaction of SPION-EGF conjugates with cells was analyzed in a C6 glioma cell culture. The distribution of the nanoparticles and their accumulation in tumors were assessed by magnetic resonance imaging in an orthotopic model of C6 gliomas. SPION-EGF nanosuspensions had the properties of a negative contrast agent with high coefficients of relaxation efficiency. In vitro studies of SPION-EGF nanoparticles showed high intracellular incorporation and the absence of a toxic influence on C6 cell viability and proliferation. Intravenous administration of SPION-EGF conjugates in animals provided receptor-mediated targeted delivery across the blood-brain barrier and tumor retention of the nanoparticles; this was more efficient than with unconjugated SPIONs. The accumulation of conjugates in the glioma was revealed as hypotensive zones on T2-weighted images with a twofold reduction in T2 relaxation time in comparison to unconjugated SPIONs (P<0.001). SPION-EGF conjugates provide targeted delivery and efficient magnetic resonance contrast enhancement of EGFR-overexpressing C6 gliomas.
Keywords: C6 glioma; EGFR; MRI contrast agent; SPION; brain tumor; epidermal growth factor; magnetic nanoparticles.
Figures


Similar articles
-
Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma.Neuro Oncol. 2014 Jan;16(1):38-49. doi: 10.1093/neuonc/not141. Epub 2013 Dec 4. Neuro Oncol. 2014. PMID: 24305705 Free PMC article.
-
Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma.Neoplasia. 2015 Jan;17(1):32-42. doi: 10.1016/j.neo.2014.11.001. Neoplasia. 2015. PMID: 25622897 Free PMC article.
-
Monoclonal antibody-conjugated superparamagnetic iron oxide nanoparticles for imaging of epidermal growth factor receptor-targeted cells and gliomas.Mol Imaging. 2015;14. doi: 10.2310/7290.2015.00002. Mol Imaging. 2015. PMID: 26044549
-
Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate.2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641831 Free Books & Documents. Review.
-
Magnetic nanoparticles for magnetic drug targeting.Biomed Tech (Berl). 2015 Oct;60(5):465-75. doi: 10.1515/bmt-2015-0049. Biomed Tech (Berl). 2015. PMID: 26351783 Review.
Cited by
-
CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field.Int J Nanomedicine. 2019 Sep 16;14:7549-7560. doi: 10.2147/IJN.S215087. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31571863 Free PMC article.
-
Seeing Better and Going Deeper in Cancer Nanotheranostics.Int J Mol Sci. 2019 Jul 16;20(14):3490. doi: 10.3390/ijms20143490. Int J Mol Sci. 2019. PMID: 31315232 Free PMC article. Review.
-
Advances in Targeted Drug Delivery Approaches for the Central Nervous System Tumors: The Inspiration of Nanobiotechnology.J Neuroimmune Pharmacol. 2017 Mar;12(1):84-98. doi: 10.1007/s11481-016-9698-1. Epub 2016 Jul 23. J Neuroimmune Pharmacol. 2017. PMID: 27449494 Review.
-
Drug Delivery Systems in the Development of Novel Strategies for Glioblastoma Treatment.Pharmaceutics. 2022 Jun 1;14(6):1189. doi: 10.3390/pharmaceutics14061189. Pharmaceutics. 2022. PMID: 35745762 Free PMC article. Review.
-
Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge.Nanomaterials (Basel). 2015 Dec 11;5(4):2231-2248. doi: 10.3390/nano5042231. Nanomaterials (Basel). 2015. PMID: 28347118 Free PMC article. Review.
References
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

