Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery
- PMID: 24421640
- PMCID: PMC3888352
- DOI: 10.2147/IJN.S51258
Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery
Abstract
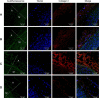
Incomplete endothelialization, blood cell adhesion to vascular stents, and inflammation of arteries can result in acute stent thromboses. The systemic administration of acetylsalicylic acid decreases endothelial dysfunction, potentially reducing thrombus, enhancing vasodilatation, and inhibiting the progression of atherosclerosis; but, this is weakened by upper gastrointestinal bleeding. This study proposes a hybrid stent with biodegradable nanofibers, for the local, sustained delivery of acetylsalicylic acid to injured artery walls. Biodegradable nanofibers are prepared by first dissolving poly(D,L)-lactide-co-glycolide and acetylsalicylic acid in 1,1,1,3,3,3-hexafluoro-2-propanol. The solution is then electrospun into nanofibrous tubes, which are then mounted onto commercially available bare-metal stents. In vitro release rates of pharmaceuticals from nanofibers are characterized using an elution method, and a highperformance liquid chromatography assay. The experimental results suggest that biodegradable nanofibers release high concentrations of acetylsalicylic acid for three weeks. The in vivo efficacy of local delivery of acetylsalicylic acid in reducing platelet and monocyte adhesion, and the minimum tissue inflammatory reaction caused by the hybrid stents in treating denuded rabbit arteries, are documented. The proposed hybrid stent, with biodegradable acetylsalicylic acid-loaded nanofibers, substantially contributed to local, sustained delivery of drugs to promote re-endothelialization and reduce thrombogenicity in the injured artery. The stents may have potential applications in the local delivery of cardiovascular drugs. Furthermore, the use of hybrid stents with acetylsalicylic acid-loaded nanofibers that have high drug loadings may provide insight into the treatment of patients with high risk of acute stent thromboses.
Keywords: acetylsalicylic acid; biodegradable drug-eluting nanofibers; cell adhesion to vascular stents; release characteristics.
Figures












Similar articles
-
Promoting endothelial recovery and reducing neointimal hyperplasia using sequential-like release of acetylsalicylic acid and paclitaxel-loaded biodegradable stents.Int J Nanomedicine. 2014 Aug 27;9:4117-33. doi: 10.2147/IJN.S67721. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25206303 Free PMC article.
-
Acceleration of re-endothelialization and inhibition of neointimal formation using hybrid biodegradable nanofibrous rosuvastatin-loaded stents.Biomaterials. 2014 May;35(15):4417-27. doi: 10.1016/j.biomaterials.2014.02.017. Epub 2014 Feb 28. Biomaterials. 2014. PMID: 24582553
-
Sustained local delivery of high-concentration vancomycin from a hybrid biodegradable, antibiotic-eluting, nanofiber-loaded endovascular prosthesis for treatment of mycotic aortic aneurysms.J Vasc Surg. 2018 Aug;68(2):597-606. doi: 10.1016/j.jvs.2017.07.142. Epub 2017 Oct 21. J Vasc Surg. 2018. PMID: 29066243
-
[Optimal platelet inhibition after coronary stent implantation. Current status].Herz. 2008 Jun;33(4):244-53. doi: 10.1007/s00059-008-3138-9. Herz. 2008. PMID: 18581073 Review. German.
-
Regeneration of vessel wall functionality and vascular restoration therapy with biodegradable stents -- current status.Curr Pharm Biotechnol. 2012 Oct;13(13):2440-8. Curr Pharm Biotechnol. 2012. PMID: 22280418 Review.
Cited by
-
Nanofibrous vildagliptin-eluting stents enhance re-endothelialization and reduce neointimal formation in diabetes: in vitro and in vivo.Int J Nanomedicine. 2019 Sep 13;14:7503-7513. doi: 10.2147/IJN.S211898. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31686818 Free PMC article.
-
Biodegradable Cable-Tie Rapamycin-eluting Stents.Sci Rep. 2017 Mar 8;7(1):111. doi: 10.1038/s41598-017-00131-w. Sci Rep. 2017. PMID: 28273914 Free PMC article.
-
Aspirin Inhibits Degenerative Changes of Aneurysmal Wall in a Rat Model.Neurochem Res. 2015 Jul;40(7):1537-45. doi: 10.1007/s11064-015-1603-4. Epub 2015 Jun 21. Neurochem Res. 2015. PMID: 26093650
-
Promoting endothelial recovery and reducing neointimal hyperplasia using sequential-like release of acetylsalicylic acid and paclitaxel-loaded biodegradable stents.Int J Nanomedicine. 2014 Aug 27;9:4117-33. doi: 10.2147/IJN.S67721. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25206303 Free PMC article.
-
Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications.Pharmaceuticals (Basel). 2021 Sep 11;14(9):921. doi: 10.3390/ph14090921. Pharmaceuticals (Basel). 2021. PMID: 34577621 Free PMC article.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. - PubMed
-
- Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52(5):333–342. - PubMed
-
- Cook S, Windecker S. Early stent thrombosis: past, present, and future. Circulation. 2009;119(5):657–659. - PubMed
-
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. - PubMed
-
- Orford JL, Lennon R, Melby S, et al. Frequency and correlates of coronary stent thrombosis in the modern era: analysis of a single center registry. J Am Coll Cardiol. 2002;40(9):1567–1572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

