Development of a novel enzyme-linked immunosorbent assay to detect anti-IgG against swine hepatitis E virus
- PMID: 24421718
- PMCID: PMC3885741
- DOI: 10.4142/jvs.2013.14.4.467
Development of a novel enzyme-linked immunosorbent assay to detect anti-IgG against swine hepatitis E virus
Abstract
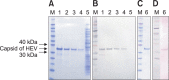
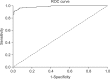
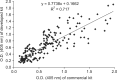
Swine hepatitis E virus (HEV) is widespread throughout pigs in both developing and industrialized countries. This virus is an important zoonotic agent and a public concern worldwide. Infected pigs are asymptomatic, so diagnosing swine HEV relies on detection of the virus or antibodies against the virus. However, several obstacles need to be overcome for effective and practical serological diagnosis. In this study, we developed an enzyme-linked immunosorbent assay (ELISA) that used a purified recombinant capsid protein of swine HEV. The potential clinical use of this assay was evaluated by comparing it with a commercial kit (Genelabs Technologies, Diagnostics, Singapore). Results of the ELISA were highly correlated with those of the commercial kit with a sensitivity of 97% and specificity of 95%. ROC (receiving operator characteristic) analysis of the ELISA data produced a value of 0.987 (95% CI, 0.977~0.998, p < 0.01). The cut-off value for the ELISA was also determined using negative pig sera. In summary, the HEV-specific ELISA developed in the present study appears to be both practical and economical.
Keywords: ELISA; diagnosis; swine hepatitis E virus.
Figures

Similar articles
-
Detection of serum antibodies to hepatitis E virus in domestic pigs in Italy using a recombinant swine HEV capsid protein.BMC Vet Res. 2014 Jun 16;10:133. doi: 10.1186/1746-6148-10-133. BMC Vet Res. 2014. PMID: 24934984 Free PMC article.
-
The use of Bayesian methods for evaluating the performance of a virus-like particles-based ELISA for serology of hepatitis E virus infection in swine.J Virol Methods. 2010 Feb;163(2):329-35. doi: 10.1016/j.jviromet.2009.10.019. Epub 2009 Oct 29. J Virol Methods. 2010. PMID: 19879297
-
Retrospective serological study on hepatitis E infection in pigs from 1985 to 1997 in Spain.Vet Microbiol. 2009 Mar 30;135(3-4):248-52. doi: 10.1016/j.vetmic.2008.09.075. Epub 2008 Oct 1. Vet Microbiol. 2009. PMID: 18996653
-
Simple and specific method for detection of antibodies against hepatitis E virus in mammalian species.J Virol Methods. 2016 Dec;238:56-61. doi: 10.1016/j.jviromet.2016.07.030. Epub 2016 Oct 11. J Virol Methods. 2016. PMID: 27732880
-
Hepatitis E virus in South America: The current scenario.Liver Int. 2018 Sep;38(9):1536-1546. doi: 10.1111/liv.13881. Epub 2018 Jun 9. Liver Int. 2018. PMID: 29788538 Review.
Cited by
-
Narrative Review of the Safety of Using Pigs for Xenotransplantation: Characteristics and Diagnostic Methods of Vertical Transmissible Viruses.Biomedicines. 2024 May 26;12(6):1181. doi: 10.3390/biomedicines12061181. Biomedicines. 2024. PMID: 38927388 Free PMC article. Review.
-
Development and Optimization of an Enzyme Immunoassay to Detect Serum Antibodies against the Hepatitis E Virus in Pigs, Using Plant-Derived ORF2 Recombinant Protein.Vaccines (Basel). 2021 Sep 6;9(9):991. doi: 10.3390/vaccines9090991. Vaccines (Basel). 2021. PMID: 34579228 Free PMC article.
-
Detection of serum antibodies to hepatitis E virus in domestic pigs in Italy using a recombinant swine HEV capsid protein.BMC Vet Res. 2014 Jun 16;10:133. doi: 10.1186/1746-6148-10-133. BMC Vet Res. 2014. PMID: 24934984 Free PMC article.
-
Seroprevalence of Getah virus in Pigs in Eastern China Determined with a Recombinant E2 Protein-Based Indirect ELISA.Viruses. 2022 Sep 30;14(10):2173. doi: 10.3390/v14102173. Viruses. 2022. PMID: 36298726 Free PMC article.
References
-
- Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2013;10:24–33. - PubMed
-
- Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, Purcell RH. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

