Adult neurogenesis modifies excitability of the dentate gyrus
- PMID: 24421758
- PMCID: PMC3872742
- DOI: 10.3389/fncir.2013.00204
Adult neurogenesis modifies excitability of the dentate gyrus
Abstract
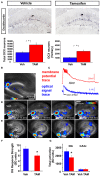
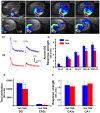
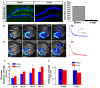
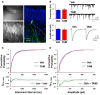
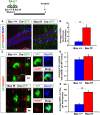
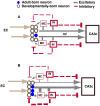
Adult-born dentate granule neurons contribute to memory encoding functions of the dentate gyrus (DG) such as pattern separation. However, local circuit-mechanisms by which adult-born neurons partake in this process are poorly understood. Computational, neuroanatomical and electrophysiological studies suggest that sparseness of activation in the granule cell layer (GCL) is conducive for pattern separation. A sparse coding scheme is thought to facilitate the distribution of similar entorhinal inputs across the GCL to decorrelate overlapping representations and minimize interference. Here we used fast voltage-sensitive dye (VSD) imaging combined with laser photostimulation and electrical stimulation to examine how selectively increasing adult DG neurogenesis influences local circuit activity and excitability. We show that DG of mice with more adult-born neurons exhibits decreased strength of neuronal activation and more restricted excitation spread in GCL while maintaining effective output to CA3c. Conversely, blockade of adult hippocampal neurogenesis changed excitability of the DG in the opposite direction. Analysis of GABAergic inhibition onto mature dentate granule neurons in the DG of mice with more adult-born neurons shows a modest readjustment of perisomatic inhibitory synaptic gain without changes in overall inhibitory tone, presynaptic properties or GABAergic innervation pattern. Retroviral labeling of connectivity in mice with more adult-born neurons showed increased number of excitatory synaptic contacts of adult-born neurons onto hilar interneurons. Together, these studies demonstrate that adult hippocampal neurogenesis modifies excitability of mature dentate granule neurons and that this non-cell autonomous effect may be mediated by local circuit mechanisms such as excitatory drive onto hilar interneurons. Modulation of DG excitability by adult-born dentate granule neurons may enhance sparse coding in the GCL to influence pattern separation.
Keywords: adult neurogenesis; dentate granule neurons; dentate gyrus; encoding; excitability; inhibition; pattern separation.
Figures