Donor/recipient enhancement of memory in rat hippocampus
- PMID: 24421759
- PMCID: PMC3872745
- DOI: 10.3389/fnsys.2013.00120
Donor/recipient enhancement of memory in rat hippocampus
Abstract
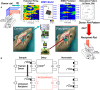
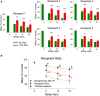
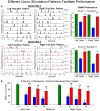
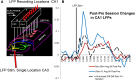
The critical role of the mammalian hippocampus in the formation, translation and retrieval of memory has been documented over many decades. There are many theories of how the hippocampus operates to encode events and a precise mechanism was recently identified in rats performing a short-term memory task which demonstrated that successful information encoding was promoted via specific patterns of activity generated within ensembles of hippocampal neurons. In the study presented here, these "representations" were extracted via a customized non-linear multi-input multi-output (MIMO) mathematical model which allowed prediction of successful performance on specific trials within the testing session. A unique feature of this characterization was demonstrated when successful information encoding patterns were derived online from well-trained "donor" animals during difficult long-delay trials and delivered via online electrical stimulation to synchronously tested naïve "recipient" animals never before exposed to the delay feature of the task. By transferring such model-derived trained (donor) animal hippocampal firing patterns via stimulation to coupled naïve recipient animals, their task performance was facilitated in a direct "donor-recipient" manner. This provides the basis for utilizing extracted appropriate neural information from one brain to induce, recover, or enhance memory related processing in the brain of another subject.
Keywords: electrical stimulation; ensemble; memory-transfer; non-linear model; rodent.
Figures


References
-
- Deadwyler S. A., Hampson R. E. (1997). The significance of neural ensemble codes during behavior and cognition, in Annual Review of Neuroscience, Vol. 20, eds Cowan W. M., Shooter E. M., Stevens C. F., Thompson R. F. (Palo Alto, CA: Annual Reviews, Inc; ), 217–244 10.1146/annurev.neuro.20.1.217 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

