A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers
- PMID: 24421886
- PMCID: PMC3885431
- DOI: 10.1371/journal.pone.0083449
A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers
Abstract
Background: Acellular pertussis vaccines do not control pertussis. A new approach to offer protection to infants is necessary. BPZE1, a genetically modified Bordetella pertussis strain, was developed as a live attenuated nasal pertussis vaccine by genetically eliminating or detoxifying 3 toxins.
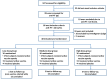
Methods: We performed a double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally for the first time to human volunteers, the first trial of a live attenuated bacterial vaccine specifically designed for the respiratory tract. 12 subjects per dose group received 10³, 10⁵ or 10⁷ colony-forming units as droplets with half of the dose in each nostril. 12 controls received the diluent. Local and systemic safety and immune responses were assessed during 6 months, and nasopharyngeal colonization with BPZE1 was determined with repeated cultures during the first 4 weeks after vaccination.
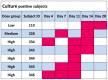
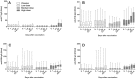
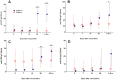
Results: Colonization was seen in one subject in the low dose, one in the medium dose and five in the high dose group. Significant increases in immune responses against pertussis antigens were seen in all colonized subjects. There was one serious adverse event not related to the vaccine. Other adverse events were trivial and occurred with similar frequency in the placebo and vaccine groups.
Conclusions: BPZE1 is safe in healthy adults and able to transiently colonize the nasopharynx. It induces immune responses in all colonized individuals. BPZE1 can thus undergo further clinical development, including dose optimization and trials in younger age groups.
Trial registration: ClinicalTrials.gov NCT01188512.
Conflict of interest statement
Figures

References
-
- World Health Organization. Vaccine-preventable diseases: monitoring system. Available at: http://www.who.int/immunization_monitoring/diseases/pertussis/en/index.html. Accessed 2013 Nov 14.
-
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J (1996) A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 334: 349–55. - PubMed
-
- World Health Organization (2011) Weekly epidemiological record. 86: 509–20.
-
- Witt MA, Katz PH, Witt DJ (2012) Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American Outbreak. Clin Infect Dis 54: 1730–5. - PubMed
-
- Sheridan SL, Ware RS, Grimwood K, Lambert SB (2012) Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308: 454–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

