Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP)
- PMID: 24421901
- PMCID: PMC3885443
- DOI: 10.1371/journal.pone.0083736
Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP)
Abstract
Background: Pre-exposure prophylaxis (PrEP) trials using tenofovir-based regimens have demonstrated that high levels of adherence are required to evaluate efficacy; the incorporation of objective biomarkers of adherence in trial design has been essential to interpretation, given the inaccuracy of self-report. Antiretroviral measurements in scalp hair have been useful as a marker of long-term exposure in the HIV treatment setting, and hair samples are relatively easy and inexpensive to collect, transport, and store for analysis. To evaluate the relationship between dose and tenofovir concentrations in hair, we examined the dose proportionality of tenofovir in hair in healthy, HIV-uninfected adults.
Methods: A phase I, crossover pharmacokinetic study was performed in 24 HIV-negative adults receiving directly-observed oral tenofovir tablets administered 2, 4, and 7 doses/week for 6 weeks, with a ≥3-week break between periods. Small samples of hair were collected after each six-week period and analyzed for tenofovir concentrations. Geometric-mean-ratios compared levels between each pair of dosing conditions. Intensive plasma pharmacokinetic studies were performed during the daily-dosing period to calculate areas-under-the-time-concentration curves (AUCs).
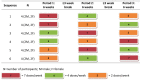
Results: Over 90% of doses were observed per protocol. Median tenofovir concentrations in hair increased monotonically with dose. A log-linear relationship was seen between dose and hair levels, with an estimated 76% (95% CI 60-93%) increase in hair level per 2-fold dose increase. Tenofovir plasma AUCs modestly predicted drug concentrations in hair.
Conclusions: This study found a strong linear relationship between frequency of dosing and tenofovir levels in scalp hair. The analysis of quantitative drug levels in hair has the potential to improve adherence measurement in the PrEP field and may be helpful in determining exposure thresholds for protection and explaining failures in PrEP trials. Hair measures for adherence monitoring may also facilitate adherence measurement in real-world settings and merit further investigation in upcoming PrEP implementation studies and programs.
Trial registration: ClinicalTrials.gov NCT00903084.
Conflict of interest statement
Figures

References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al. (2012) Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367: 423–434. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, et al. (2013) Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381: 2083–2090. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

