The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid
- PMID: 24421907
- PMCID: PMC3885440
- DOI: 10.1371/journal.pone.0083828
The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid
Abstract
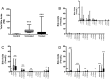
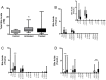
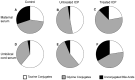
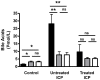
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver disorder associated with an increased risk of adverse fetal outcomes. It is characterised by raised maternal serum bile acids, which are believed to cause the adverse outcomes. ICP is commonly treated with ursodeoxycholic acid (UDCA). This study aimed to determine the fetal and maternal bile acid profiles in normal and ICP pregnancies, and to examine the effect of UDCA treatment. Matched maternal and umbilical cord serum samples were collected from untreated ICP (n = 18), UDCA-treated ICP (n = 46) and uncomplicated pregnancy (n = 15) cases at the time of delivery. Nineteen individual bile acids were measured using HPLC-MS/MS. Maternal and fetal serum bile acids are significantly raised in ICP compared with normal pregnancy (p = <0.0001 and <0.05, respectively), predominantly due to increased levels of conjugated cholic and chenodeoxycholic acid. There are no differences between the umbilical cord artery and cord vein levels of the major bile acid species. The feto-maternal gradient of bile acids is reversed in ICP. Treatment with UDCA significantly reduces serum bile acids in the maternal compartment (p = <0.0001), thereby reducing the feto-maternal transplacental gradient. UDCA-treatment does not cause a clinically important increase in lithocholic acid (LCA) concentrations. ICP is associated with significant quantitative and qualitative changes in the maternal and fetal bile acid pools. Treatment with UDCA reduces the level of bile acids in both compartments and reverses the qualitative changes. We have not found evidence to support the suggestion that UDCA treatment increases fetal LCA concentrations to deleterious levels.
Conflict of interest statement
Figures
Similar articles
-
Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations.J Hepatol. 2021 May;74(5):1087-1096. doi: 10.1016/j.jhep.2020.11.038. Epub 2020 Dec 1. J Hepatol. 2021. PMID: 33276032 Free PMC article.
-
Unresolved alterations in bile acid composition and dyslipidemia in maternal and cord blood after UDCA treatment for intrahepatic cholestasis of pregnancy.Am J Physiol Gastrointest Liver Physiol. 2025 Apr 1;328(4):G364-G376. doi: 10.1152/ajpgi.00266.2024. Epub 2025 Feb 13. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 39947696
-
Enzymatic quantification of total serum bile acids as a monitoring strategy for women with intrahepatic cholestasis of pregnancy receiving ursodeoxycholic acid treatment: a cohort study.BJOG. 2019 Dec;126(13):1633-1640. doi: 10.1111/1471-0528.15926. Epub 2019 Sep 26. BJOG. 2019. PMID: 31483939 Free PMC article.
-
Intrahepatic cholestasis of pregnancy: changes in maternal-fetal bile acid balance and improvement by ursodeoxycholic acid.Ann Hepatol. 2002 Jan-Mar;1(1):20-8. Ann Hepatol. 2002. PMID: 15114292 Review.
-
Evaluating the effectiveness and safety of ursodeoxycholic acid in treatment of intrahepatic cholestasis of pregnancy: A meta-analysis (a prisma-compliant study).Medicine (Baltimore). 2016 Oct;95(40):e4949. doi: 10.1097/MD.0000000000004949. Medicine (Baltimore). 2016. PMID: 27749550 Free PMC article. Review.
Cited by
-
Estrone sulphate uptake by the microvillous membrane of placental syncytiotrophoblast is coupled to glutamate efflux.Biochem Biophys Res Commun. 2018 Nov 17;506(1):237-242. doi: 10.1016/j.bbrc.2018.10.074. Epub 2018 Oct 19. Biochem Biophys Res Commun. 2018. PMID: 30343886 Free PMC article.
-
Causal association between gut microbiota and intrahepatic cholestasis of pregnancy: mendelian randomization study.BMC Pregnancy Childbirth. 2023 Aug 5;23(1):568. doi: 10.1186/s12884-023-05889-8. BMC Pregnancy Childbirth. 2023. PMID: 37543573 Free PMC article.
-
Dysregulated Hepatic Expression of Glucose Transporter Type-1, Toll-Like Receptor 4, and Nuclear Factor Kappa B in Estrogen-Induced Cholestasis Pregnant Rats with Placental Ischemia-Reperfusion Stress.Matern Fetal Med. 2020 Nov 17;4(1):17-23. doi: 10.1097/FM9.0000000000000079. eCollection 2022 Jan. Matern Fetal Med. 2020. PMID: 40406577 Free PMC article.
-
From dried bear bile to molecular investigation of differential effects of bile acids in ex vivo and in vitro models of myocardial dysfunction: Relevance for neuroinflammation.Brain Behav Immun Health. 2023 Aug 2;32:100674. doi: 10.1016/j.bbih.2023.100674. eCollection 2023 Oct. Brain Behav Immun Health. 2023. PMID: 37593199 Free PMC article. Review.
-
Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs.Nat Rev Endocrinol. 2019 Dec;15(12):701-712. doi: 10.1038/s41574-019-0266-7. Epub 2019 Oct 15. Nat Rev Endocrinol. 2019. PMID: 31616073 Free PMC article. Review.
References
-
- Macias RI, Pascual MJ, Bravo A, Alcalde MP, Larena MG, et al. (2000) Effect of maternal cholestasis on bile acid transfer across the rat placenta-maternal liver tandem. Hepatology 31: 975–983. - PubMed
-
- Sewell RB, Hardy KJ, Smallwood RA, Hoffman NE (1980) Fetal bile salt metabolism: placental transfer of taurocholate in sheep. Am J Physiol 239: G354–357. - PubMed
-
- Sewell RB, Hardy KJ, Smallwood RA, Hoffman NE (1982) Fetal bile salt metabolism: placental transfer of dihydroxy bile salts in sheep. Am J Physiol 243: G172–175. - PubMed
-
- Cui T, Liu Y, Men X, Xu Z, Wu L, et al. (2009) Bile acid transport correlative protein mRNA expression profile in human placenta with intrahepatic cholestasis of pregnancy. Saudi Med J 30: 1406–1410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

