Clinical outcomes and toxicity of proton beam therapy for advanced cholangiocarcinoma
- PMID: 24422711
- PMCID: PMC3904195
- DOI: 10.1186/1748-717X-9-26
Clinical outcomes and toxicity of proton beam therapy for advanced cholangiocarcinoma
Abstract
Background: We examined the efficacy and toxicity of proton beam therapy (PBT) for treating advanced cholangiocarcinoma.
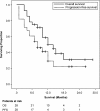
Methods: The clinical data and outcomes of 28 cholangiocarcinoma patients treated with PBT between January 2009 and August 2011 were retrospectively examined. The Kaplan-Meier method was used to estimate overall survival (OS), progression-free survival (PFS), and local control (LC) rates, and the log-rank test to analyze the effects of different clinical and treatment variables on survival. Acute and late toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Results: The median age of the 17 male and 11 female patients was 71 years (range, 41 to 84 years; intrahepatic/peripheral cholangiocarcinoma, n = 6; hilar cholangiocarcinoma/Klatskin tumor, n = 6; distal extrahepatic cholangiocarcinoma, n = 3; gallbladder cancer, n = 3; local or lymph node recurrence, n = 10; size, 20-175 mm; median 52 mm). The median radiation dose was 68.2 Gy (relative biological effectiveness [RBE]) (range, 50.6 to 80 Gy (RBE)), with delivery of fractions of 2.0 to 3.2 Gy (RBE) daily. The median follow-up duration was 12 months (range, 3 to 29 months). Fifteen patients underwent chemotherapy and 8 patients, palliative biliary stent placement prior to PBT. OS, PFS, and LC rates at 1 year were 49.0%, 29.5%, and 67.7%, respectively. LC was achieved in 6 patients, and was better in patients administered a biologically equivalent dose of 10 (BED10) > 70 Gy compared to those administered < 70 Gy (83.1% vs. 22.2%, respectively, at 1 year). The variables of tumor size and performance status were associated with survival. Late gastrointestinal toxicities grade 2 or greater were observed in 7 patients <12 months after PBT. Cholangitis was observed in 11 patients and 3 patients required stent replacement.
Conclusions: Relatively high LC rates after PBT for advanced cholangiocarcinoma can be achieved by delivery of a BED10 > 70 Gy. Gastrointestinal toxicities, especially those of the duodenum, are dose-limiting toxicities associated with PBT, and early metastatic progression remains a treatment obstacle.
Figures
Similar articles
-
Role and Effectiveness of Hypofractionated Proton Beam Therapy and Combinations with Systemic Chemotherapy in Inoperable Extrahepatic Cholangiocarcinoma.Cancer Res Treat. 2025 Jul;57(3):852-864. doi: 10.4143/crt.2024.805. Epub 2024 Dec 17. Cancer Res Treat. 2025. PMID: 39701089 Free PMC article.
-
Updated Japanese multicenter registry study evaluates the efficacy and safety of proton beam therapy for treating extrahepatic cholangiocarcinoma.Sci Rep. 2025 Jul 2;15(1):23250. doi: 10.1038/s41598-025-06575-9. Sci Rep. 2025. PMID: 40603425 Free PMC article.
-
Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma.BMC Cancer. 2017 Nov 21;17(1):781. doi: 10.1186/s12885-017-3788-1. BMC Cancer. 2017. PMID: 29162055 Free PMC article.
-
Proton Beam Therapy in Liver Malignancies.Curr Oncol Rep. 2020 Feb 27;22(3):30. doi: 10.1007/s11912-020-0889-9. Curr Oncol Rep. 2020. PMID: 32108284 Review.
-
Efficacy of stereotactic body radiotherapy for unresectable or recurrent cholangiocarcinoma: a meta-analysis and systematic review.Strahlenther Onkol. 2019 Feb;195(2):93-102. doi: 10.1007/s00066-018-1367-2. Epub 2018 Sep 11. Strahlenther Onkol. 2019. PMID: 30206644 English.
Cited by
-
Proton beam stereotactic body radiotherapy and hypofractionated therapy with pencil beam scanning is safe and effective for advanced hepatocellular carcinoma and intrahepatic cholangiocarcinoma: A single center experience.J Radiosurg SBRT. 2023;9(1):43-52. J Radiosurg SBRT. 2023. PMID: 38029012 Free PMC article.
-
Particle Beam Therapy for Intrahepatic and Extrahepatic Biliary Duct Carcinoma: A Multi-Institutional Retrospective Data Analysis.Cancers (Basel). 2022 Nov 28;14(23):5864. doi: 10.3390/cancers14235864. Cancers (Basel). 2022. PMID: 36497346 Free PMC article.
-
Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma.Liver Cancer. 2022 Feb 23;11(4):290-314. doi: 10.1159/000522403. eCollection 2022 Jul. Liver Cancer. 2022. PMID: 35978598 Free PMC article.
-
Particle beam therapy versus photon radiotherapy for extrahepatic biliary cancer-systemic review and meta-analysis.J Radiat Res. 2023 Jun 16;64(Supplement_1):i34-i40. doi: 10.1093/jrr/rrad015. J Radiat Res. 2023. PMID: 37036780 Free PMC article.
-
Reviewing the potential role of radiation therapy in gallbladder cancer: an update.Radiat Oncol J. 2022 Mar;40(1):1-8. doi: 10.3857/roj.2021.00717. Epub 2022 Jan 25. Radiat Oncol J. 2022. PMID: 35368195 Free PMC article.
References
-
- Parkin DM, Whelan SL, Ferlay J. et al.Cancer incidence in five continents, volumes I to VIII. IARC Cancer Base. 2005;7:258–269.
-
- Blumgart LH, Hadjis NS, Bnjamin IS. et al.Surgical approaches to cholangiocarcinoma at confluence of hepatic ducts. Lancet. 1984;1:66–70. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical