Pharmacokinetic study and evaluation of the safety of taurolidine for dogs with osteosarcoma
- PMID: 24422857
- PMCID: PMC3852505
- DOI: 10.1186/1756-9966-32-74
Pharmacokinetic study and evaluation of the safety of taurolidine for dogs with osteosarcoma
Abstract
Background: Osteosarcoma in dogs and humans share many similarities and the dog has been described as an excellent model to study this disease. The median survival in dogs has not improved in the last 25 years. Taurolidine has been shown to be cytotoxic to canine and human osteosarcoma in vitro. The goals of this study were to determine the pharmacokinetics and safety of taurolidine in healthy dogs and the safety of taurolidine in combination with doxorubicin or carboplatin in dogs with osteosarcoma.
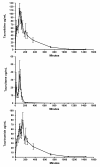
Methods: Two percent taurolidine was infused into six healthy dogs (150 mg/kg) over a period of two hours and blood samples were taken periodically. One dog received taurolidine with polyvinylpyrrolidone (PVP) as its carrier and later received PVP-free taurolidine as did all other dogs in this study. Serum taurolidine concentrations were determined using high-performance liquid chromatography (HPLC) online coupled to ESI-MS/MS in the multiple reaction monitoring mode. Subsequently, the same dose of taurolidine was infused to seven dogs with osteosarcoma also treated with doxorubicin or carboplatin.
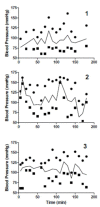
Results: Taurolidine infusion was safe in 6 healthy dogs and there were no significant side effects. Maximum taurolidine serum concentrations ranged between 229 to 646 μM. The dog that received taurolidine with PVP had an immediate allergic reaction but recovered fully after the infusion was stopped. Three additional dogs with osteosarcoma received doxorubicin and taurolidine without PVP. Toxicities included dilated cardiomyopathy, protein-losing nephropathy, renal insufficiency and vasculopathy at the injection site. One dog was switched to carboplatin instead of doxorubicin and an additional 4 dogs with osteosarcoma received taurolidine-carboplatin combination. One incidence of ototoxicity occurred with the taurolidine- carboplatin combination. Bone marrow and gastro-intestinal toxicity did not appear increased with taurolidine over doxorubicin or carboplatin alone.
Conclusions: Taurolidine did not substantially exacerbate bone marrow or gastro-intestinal toxicity however, it is possible that taurolidine increased other toxicities of doxorubicin and carboplatin. Administering taurolidine in combination with 30 mg/m2 doxorubicin in dogs is not recommended but taurolidine in combination with carboplatin (300 mg/m2) appears safe.
Figures
Similar articles
-
The effects of taurolidine alone and in combination with doxorubicin or carboplatin in canine osteosarcoma in vitro.BMC Vet Res. 2013 Jan 18;9:15. doi: 10.1186/1746-6148-9-15. BMC Vet Res. 2013. PMID: 23331343 Free PMC article.
-
Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial.Vet Comp Oncol. 2016 Mar;14(1):81-7. doi: 10.1111/vco.12069. Epub 2013 Oct 4. Vet Comp Oncol. 2016. PMID: 24118677 Free PMC article. Clinical Trial.
-
Evaluation of toxicities from combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma.J Small Anim Pract. 2014 Jul;55(7):369-74. doi: 10.1111/jsap.12228. Epub 2014 May 7. J Small Anim Pract. 2014. PMID: 24803081
-
Update on the biology and management of canine osteosarcoma.Vet Clin North Am Small Anim Pract. 2003 May;33(3):491-516, vi. doi: 10.1016/s0195-5616(03)00021-4. Vet Clin North Am Small Anim Pract. 2003. PMID: 12852233 Review.
-
Pharmacogenetics of chemotherapy treatment response and -toxicities in patients with osteosarcoma: a systematic review.BMC Cancer. 2022 Dec 19;22(1):1326. doi: 10.1186/s12885-022-10434-5. BMC Cancer. 2022. PMID: 36536332 Free PMC article.
Cited by
-
Identification of GPC3 mutation and upregulation in a multidrug resistant osteosarcoma and its spheroids as therapeutic target.J Bone Oncol. 2021 Sep 20;30:100391. doi: 10.1016/j.jbo.2021.100391. eCollection 2021 Oct. J Bone Oncol. 2021. PMID: 34611509 Free PMC article.
-
Taurultam shows antiviral activity against SARS-CoV-2 and influenza virus.BMC Microbiol. 2025 May 15;25(1):292. doi: 10.1186/s12866-025-03847-2. BMC Microbiol. 2025. PMID: 40375181 Free PMC article.
-
Exploring the effects of taurolidine on tumor weight and microvessel density in a murine model of osteosarcoma.Oncol Res. 2024 Jun 20;32(7):1163-1172. doi: 10.32604/or.2024.050907. eCollection 2024. Oncol Res. 2024. PMID: 38948019 Free PMC article.
-
The autophagy inhibitor spautin-1, either alone or combined with doxorubicin, decreases cell survival and colony formation in canine appendicular osteosarcoma cells.PLoS One. 2018 Oct 29;13(10):e0206427. doi: 10.1371/journal.pone.0206427. eCollection 2018. PLoS One. 2018. PMID: 30372478 Free PMC article.
-
Evaluation of the safety and efficacy of TRAIL and taurolidine use on human fibrosarcoma xenografts in vivo.Oncol Lett. 2016 Mar;11(3):1955-1961. doi: 10.3892/ol.2016.4118. Epub 2016 Jan 15. Oncol Lett. 2016. PMID: 26998107 Free PMC article.
References
-
- Liptak JMDW, Ehrhart N, Withrow SJ. Canine appendicular osteosarcoma: diagnosis and palliative treatment. Compend Contin Educ. 2004;26:172–182.
-
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27(1A):155–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

