Adoptive immunotherapy for cancer or viruses
- PMID: 24423116
- PMCID: PMC4533835
- DOI: 10.1146/annurev-immunol-032713-120136
Adoptive immunotherapy for cancer or viruses
Abstract
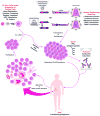
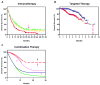
Adoptive immunotherapy, or the infusion of lymphocytes, is a promising approach for the treatment of cancer and certain chronic viral infections. The application of the principles of synthetic biology to enhance T cell function has resulted in substantial increases in clinical efficacy. The primary challenge to the field is to identify tumor-specific targets to avoid off-tumor, on-target toxicity. Given recent advances in efficacy in numerous pilot trials, the next steps in clinical development will require multicenter trials to establish adoptive immunotherapy as a mainstream technology.
Figures




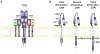

References
-
- Anderson WF. Prospects for human gene therapy. Science. 1984;226:401–9. - PubMed
-
- Friedmann T. A brief history of gene therapy. Nat Genet. 1992;2:93–8. - PubMed
-
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–93. - PubMed
-
- Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C, June CH, Lazar R, Broad DF, Jalali S, Hege KM. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5:788–97. - PubMed
-
- Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

