Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis
- PMID: 24423138
- PMCID: PMC3979102
- DOI: 10.1186/ar4436
Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis
Abstract
Introduction: Animal models currently used in osteoarthritis-associated pain research inadequately reproduce the initiating events and structural pathology of human osteoarthritis. Conversely, intra-articular injection of collagenase is a structurally relevant model, as it induces articular degeneration both by digesting collagen from cartilage and by causing articular instability, thereby reproducing some of the main events associated with osteoarthritis onset and development. Here, we evaluated if the intra-articular injection of collagenase can be an alternative model to study nociception associated with osteoarthritis.
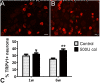
Methods: Osteoarthritis was induced by two intra-articular injections of either 250 U or 500 U of collagenase into the left knee joint of adult male Wistar rats. A six weeks time-course assessment of movement- and loading-induced nociception was performed by the Knee-Bend and CatWalk tests. The effect of morphine, lidocaine and diclofenac on nociceptive behaviour was evaluated in animals injected with 500 U of collagenase. Joint histopathology was scored for both doses throughout time. The expression of transient receptor potential vanilloid 1 (TRPV1) in ipsilateral dorsal root ganglia (DRG) was evaluated.
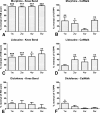
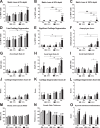
Results: An increase in nociceptive behaviour associated with movement and loading of affected joints was observed after intra-articular collagenase injection. With the 500 U dose of collagenase, there was a significant correlation between the behavioural and the histopathological osteoarthritis-like structural changes developed after six weeks. One week after injection of 500 U collagenase, swelling of the injected knee and inflammation of the synovial membrane were also observed, indicating the occurrence of an early inflammatory reaction. Behavioural changes induced by the 500 U dose of collagenase were overall effectively reversed by morphine and lidocaine. Diclofenac was effective one week after injection. TRPV1 expression increased six weeks after 500 U collagenase injection.
Conclusion: We conclude that the intra-articular injection of 500 U collagenase in the knee of rats can be an alternative model for the study of nociception associated with osteoarthritis, since it induces significant nociceptive alterations associated with relevant osteoarthritis-like joint structural changes.
Figures





References
-
- WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;16:i–x. 1–218, back cover. - PubMed
-
- Creamer P, Lethbridge-Cejku M, Hochberg MC. Where does it hurt? Pain localization in osteoarthritis of the knee. Osteoarthritis Cartilage. 1998;16:318–323. - PubMed
-
- Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol. 2006;16:537–547. - PubMed
-
- Bendele A. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;16:363–376. - PubMed
-
- Bendele A. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;16:501–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

