Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial
- PMID: 24423301
- PMCID: PMC4009579
- DOI: 10.1210/jc.2013-3318
Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial
Abstract
Context: Methodological shortcomings often compromise investigations into the effects of primary somatostatin-analog treatment on tumor size in acromegaly. There are also limited data for the long-acting lanreotide formulation.
Objective: The aim of the study was to better characterize the effects of primary lanreotide Autogel treatment on tumor size in patients with GH-secreting macroadenomas.
Design: PRIMARYS was a 48-week, multicenter, open-label, single-arm study.
Setting: The study was conducted at specialist endocrine centers.
Patients: Treatment-naïve acromegalic patients with GH-secreting macroadenomas participated in the study.
Intervention: Lanreotide Autogel 120 mg was administered sc every 28 days (without dose titration).
Outcome measures: The primary endpoint was the proportion of patients with clinically significant (≥20%) tumor volume reduction (TVR) at week 48/last post-baseline value available using central assessments from three readers. The null hypothesis (H0) for the primary endpoint was that the proportion with TVR was ≤55%. Secondary endpoints included: TVR at other time points, GH and IGF-1, acromegalic symptoms, quality of life (QoL), and safety.
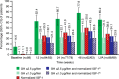
Results: Sixty-four of 90 (71.1%) patients completed the study. Clinically significant TVR at 48 weeks/last post-baseline value available was achieved by 62.9% (95% confidence interval, 52.0, 72.9) of 89 patients in the primary analysis (intention-to-treat population; H0 not rejected) and 71.9-75.3% in sensitivity (n = 89) and secondary analyses (n = 63) (H0 rejected). At 12 weeks, 54.1% had clinically significant TVR. Early and sustained improvements also occurred in GH and IGF-1, acromegalic symptoms, and QoL. No patients withdrew due to gastrointestinal intolerance.
Conclusions: Primary treatment with lanreotide Autogel, administered at 120 mg (highest available dose) without dose titration, in patients with GH-secreting macroadenomas provides early and sustained reductions in tumor volume, GH and IGF-1, and acromegalic symptoms, and improves QoL.
Trial registration: ClinicalTrials.gov NCT00690898.
Figures



References
-
- Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011;17(suppl 4):1–44 - PubMed
-
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol. 2005;152:379–387 - PubMed
-
- Melmed S, Colao A, Barkan A, et al. ; Acromegaly Consensus Group. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–1517 - PubMed
-
- Giustina A, Bronstein MD, Casanueva FF, et al. . Current management practices for acromegaly: an international survey. Pituitary. 2011;14:125–133 - PubMed
-
- Colao A, Auriemma RS, Rebora A, et al. . Significant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol (Oxf). 2009;71:237–245 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

