Pasireotide versus octreotide in acromegaly: a head-to-head superiority study
- PMID: 24423324
- PMCID: PMC3965714
- DOI: 10.1210/jc.2013-2480
Pasireotide versus octreotide in acromegaly: a head-to-head superiority study
Abstract
Context: Biochemical control reduces morbidity and increases life expectancy in patients with acromegaly. With current medical therapies, including the gold standard octreotide long-acting-release (LAR), many patients do not achieve biochemical control.
Objective: Our objective was to demonstrate the superiority of pasireotide LAR over octreotide LAR in medically naive patients with acromegaly.
Design and setting: We conducted a prospective, randomized, double-blind study at 84 sites in 27 countries.
Patients: A total of 358 patients with medically naive acromegaly (GH >5 μg/L or GH nadir ≥1 μg/L after an oral glucose tolerance test (OGTT) and IGF-1 above the upper limit of normal) were enrolled. Patients either had previous pituitary surgery but no medical treatment or were de novo with a visible pituitary adenoma on magnetic resonance imaging.
Interventions: Patients received pasireotide LAR 40 mg/28 days (n = 176) or octreotide LAR 20 mg/28 days (n = 182) for 12 months. At months 3 and 7, titration to pasireotide LAR 60 mg or octreotide LAR 30 mg was permitted, but not mandatory, if GH ≥2.5μg/L and/or IGF-1 was above the upper limit of normal.
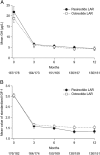
Main outcome measure: The main outcome measure was the proportion of patients in each treatment arm with biochemical control (GH <2.5 μg/L and normal IGF-1) at month 12.
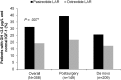
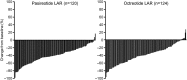
Results: Biochemical control was achieved by significantly more pasireotide LAR patients than octreotide LAR patients (31.3% vs 19.2%; P = .007; 35.8% vs 20.9% when including patients with IGF-1 below the lower normal limit). In pasireotide LAR and octreotide LAR patients, respectively, 38.6% and 23.6% (P = .002) achieved normal IGF-1, and 48.3% and 51.6% achieved GH <2.5 μg/L. 31.0% of pasireotide LAR and 22.2% of octreotide LAR patients who did not achieve biochemical control did not receive the recommended dose increase. Hyperglycemia-related adverse events were more common with pasireotide LAR (57.3% vs 21.7%).
Conclusions: Pasireotide LAR demonstrated superior efficacy over octreotide LAR and is a viable new treatment option for acromegaly.
Figures
References
-
- Arosio M, Reimondo G, Malchiodi E, et al. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol. 2012;167:189–198 - PubMed
-
- Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95 - PubMed
-
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol. 2005;152:379–387 - PubMed
-
- Grasso LF, Pivonello R, Colao A. Somatostatin analogs as a first-line treatment in acromegaly: when is it appropriate? Curr Opin Endocrinol Diabetes Obes. 2012;19:288–294 - PubMed
-
- Colao A, Cappabianca P, Caron P, et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin Endocrinol (Oxf). 2009;70:757–768 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

