21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis
- PMID: 24423334
- PMCID: PMC3973780
- DOI: 10.1210/jc.2013-3204
21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis
Abstract
Context: Mineralocorticoid synthesis by the nonhuman primate periovulatory follicle enhances luteinization. Whether a similar event occurs in women undergoing in vitro fertilization (IVF) is unknown.
Objective: The objective of the study was to determine whether human luteinized granulosa cells (LGCs) produce mineralocorticoids derived from 21-hydroxylase activity and also express mRNA for 21-hydroxylase and the mineralocorticoid receptor.
Design: This was a prospective cohort study.
Setting: The study was conducted at an academic center.
Patients: LGC lipid content and follicle fluid (FF) hormone analysis was performed on 27 nonobese IVF women. LGCs from six additional nonobese IVF women were used for gene expression studies.
Intervention: At oocyte retrieval, FF was aspirated from the first follicle (≥16 mm in size) of each ovary and pooled LGCs were collected.
Main outcome measures: FF steroid analysis was performed by liquid chromatography-tandem mass spectrometry. LGCs were stained with lipid fluorescent dye BODIPY FL C16 to estimate lipid content by confocal microscopy as a cholesterol source for steroidogenesis in vivo. Quantitative real-time PCR was performed using LGCs to detect 21-hydroxylase and mineralocorticoid receptor mRNA expression. Pearson correlation coefficients determined associations between FF steroid levels and LGC lipid content.
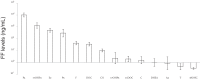
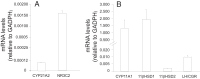
Results: FF levels of the 21-hydroxylase-derived steroids, 11-deoxycorticosterone [DOC, 39.97, median (13.94-63.02) ng/mL] and 11-deoxycortisol [11DOC, 2.07 (0.69-5.01) ng/mL], along with the 21-hydroxylase precursor 17-hydroxyprogesterone [1268.21 (493.26-3558.39) ng/mL], positively correlated with LGC lipid content (84 ± 43 fluorescent units/sample) (P ≤ .05, all steroids). 21-Hydroxylase and mineralocorticoid receptor mRNA expression was detected in LGCs.
Conclusions: Human LGCs likely synthesize 21-hydroxylase-derived mineralocorticoids from cholesterol-containing lipid in vivo to promote postovulatory luteinization via mineralocorticoid receptor-mediated events.
Figures


References
-
- Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab. 1998;83:983–991 - PubMed
-
- Parinaud J, Perret B, Ribbes H, Chap H, Pontonnier G, Douste-Blazy L. High density lipoprotein and low density lipoprotein utilization by human granulosa cells for progesterone synthesis in serum-free culture: respective contributions of free and esterified cholesterol. J Clin Endocrinol Metab. 1987;64:409–417 - PubMed
-
- Rajan VP, Menon KM. Cholesterol flux between high density lipoproteins and cultured rat luteal cells. Endocrinology. 1989;124:1857–1862 - PubMed
-
- Salway JG. Metabolism at a Glance. 3rd ed Malden, MA: Blackwell Publishing; 2010:72
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

