Critical appraisal of quantitative PCR results in colorectal cancer research: can we rely on published qPCR results?
- PMID: 24423493
- PMCID: PMC5528534
- DOI: 10.1016/j.molonc.2013.12.016
Critical appraisal of quantitative PCR results in colorectal cancer research: can we rely on published qPCR results?
Abstract
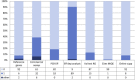
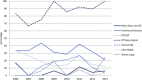
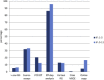
The use of real-time quantitative polymerase chain reaction (qPCR) in cancer research has become ubiquitous. The relative simplicity of qPCR experiments, which deliver fast and cost-effective results, means that each year an increasing number of papers utilizing this technique are being published. But how reliable are the published results? Since the validity of gene expression data is greatly dependent on appropriate normalisation to compensate for sample-to-sample and run-to-run variation, we have evaluated the adequacy of normalisation procedures in qPCR-based experiments. Consequently, we assessed all colorectal cancer publications that made use of qPCR from 2006 until August 2013 for the number of reference genes used and whether they had been validated. Using even these minimal evaluation criteria, the validity of only three percent (6/179) of the publications can be adequately assessed. We describe common errors, and conclude that the current state of reporting on qPCR in colorectal cancer research is disquieting. Extrapolated to the study of cancer in general, it is clear that the majority of studies using qPCR cannot be reliably assessed and that at best, the results of these studies may or may not be valid and at worst, pervasive incorrect normalisation is resulting in the wholesale publication of incorrect conclusions. This survey demonstrates that the existence of guidelines, such as MIQE, is necessary but not sufficient to address this problem and suggests that the scientific community should examine its responsibility and be aware of the implications of these findings for current and future research.
Keywords: Colorectal cancer; MIQE; Real time PCR; qPCR.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Andersen, C.L. , Lamy, P. , Thorsen, K. , Kjeldsen, E. , Wikman, F. , Villesen, P. , Oster, B. , Laurberg, S. , Orntoft, T.F. , 2011. Frequent genomic loss at chr16p13.2 is associated with poor prognosis in colorectal cancer. Int. J. Cancer. 129, 1848–1858. - PubMed
-
- Artells, R. , Moreno, I. , Diaz, T. , Martinez, F. , Gel, B. , Navarro, A. , Ibeas, R. , Moreno, J. , Monzo, M. , 2010. Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients. Eur. J. Cancer. 46, 642–649. - PubMed
-
- Bustin, S.A. , 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol.. 25, 169–193. - PubMed
-
- Bustin, S.A. , 2010. Why the need for qPCR publication guidelines?–The case for MIQE. Methods. 50, 217–226. - PubMed
-
- Bustin, S.A. , Benes, V. , Garson, J. , Hellemans, J. , Huggett, J. , Kubista, M. , Mueller, R. , Nolan, T. , Pfaffl, M.W. , Shipley, G. , Wittwer, C.T. , Schjerling, P. , Day, P.J. , Abreu, M. , Aguado, B. , Beaulieu, J.F. , Beckers, A. , Bogaert, S. , Browne, J.A. , Carrasco-Ramiro, F. , Ceelen, L. , Ciborowski, K. , Cornillie, P. , Coulon, S. , Cuypers, A. , De Brouwer, S. , De Ceuninck, L. , De Craene, J. , De Naeyer, H. , De Spiegelaere, W. , Deckers, K. , Dheedene, A. , Durinck, K. , Ferreira-Teixeira, M. , Fieuw, A. , Gallup, J.M. , Gonzalo-Flores, S. , Goossens, K. , Heindryckx, F. , Herring, E. , Hoenicka, H. , Icardi, L. , Jaggi, R. , Javad, F. , Karampelias, M. , Kibenge, F. , Kibenge, M. , Kumps, C. , Lambertz, I. , Lammens, T. , Markey, A. , Messiaen, P. , Mets, E. , Morais, S. , Mudarra-Rubio, A. , Nakiwala, J. , Nelis, H. , Olsvik, P.A. , Perez-Novo, C. , Plusquin, M. , Remans, T. , Rihani, A. , Rodrigues-Santos, P. , Rondou, P. , Sanders, R. , Schmidt-Bleek, K. , Skovgaard, K. , Smeets, K. , Tabera, L. , Toegel, S. , Van Acker, T. , Van den Broeck, W. , Van der Meulen, J. , Van Gele, M. , Van Peer, G. , Van Poucke, M. , Van Roy, N. , Vergult, S. , Wauman, J. , Tshuikina-Wiklander, M. , Willems, E. , Zaccara, S. , Zeka, F. , Vandesompele, J. , 2013. The need for transparency and good practices in the qPCR literature. Nat. Methods. 10, 1063–1067. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

