Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats
- PMID: 24423923
- PMCID: PMC3950859
- DOI: 10.1038/bjc.2013.830
Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats
Abstract
Background: In experimental models of glioblastoma multiforme (GBM), irradiation (IR) induces local expression of the chemokine CXCL12/SDF-1, which promotes tumour recurrence. The role of CXCR7, the high-affinity receptor for CXCL12, in the tumour's response to IR has not been addressed.
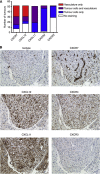
Methods: We tested CXCR7 inhibitors for their effects on tumour growth and/or animal survival post IR in three rodent GBM models. We used immunohistochemistry to determine where CXCR7 protein is expressed in the tumours and in human GBM samples. We used neurosphere formation assays with human GBM xenografts to determine whether CXCR7 is required for cancer stem cell (CSC) activity in vitro.
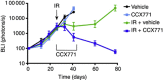
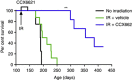
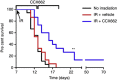
Results: CXCR7 was detected on tumour cells and/or tumour-associated vasculature in the rodent models and in human GBM. In human GBM, CXCR7 expression increased with glioma grade and was spatially associated with CXCL12 and CXCL11/I-TAC. In the rodent GBM models, pharmacological inhibition of CXCR7 post IR caused tumour regression, blocked tumour recurrence, and/or substantially prolonged survival. CXCR7 expression levels on human GBM xenograft cells correlated with neurosphere-forming activity, and a CXCR7 inhibitor blocked sphere formation by sorted CSCs.
Conclusions: These results indicate that CXCR7 inhibitors could block GBM tumour recurrence after IR, perhaps by interfering with CSCs.
Figures




References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
-
- Berahovich RD, Wang Y, Jaen JC, Schall TJ. CXCR7 protein is not expressed on human or mouse leukocytes. J Immunol. 2010;185:5130–5139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

