Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions
- PMID: 24423980
- PMCID: PMC3963261
- DOI: 10.1210/er.2013-1087
Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions
Abstract
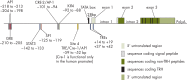
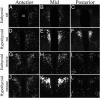
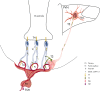
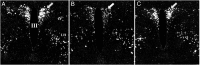
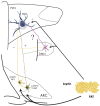
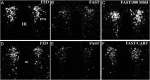
TRH is a tripeptide amide that functions as a neurotransmitter but also serves as a neurohormone that has a critical role in the central regulation of the hypothalamic-pituitary-thyroid axis. Hypophysiotropic TRH neurons involved in this neuroendocrine process are located in the hypothalamic paraventricular nucleus and secrete TRH into the pericapillary space of the external zone of the median eminence for conveyance to anterior pituitary thyrotrophs. Under basal conditions, the activity of hypophysiotropic TRH neurons is regulated by the negative feedback effects of thyroid hormone to ensure stable, circulating, thyroid hormone concentrations, a mechanism that involves complex interactions between hypophysiotropic TRH neurons and the vascular system, cerebrospinal fluid, and specialized glial cells called tanycytes. Hypophysiotropic TRH neurons also integrate other humoral and neuronal inputs that can alter the setpoint for negative feedback regulation by thyroid hormone. This mechanism facilitates adaptation of the organism to changing environmental conditions, including the shortage of food and a cold environment. The thyroid axis is also affected by other adverse conditions such as infection, but the central mechanisms mediating suppression of hypophysiotropic TRH may be pathophysiological. In this review, we discuss current knowledge about the mechanisms that contribute to the regulation of hypophysiotropic TRH neurons under physiological and pathophysiological conditions.
Figures









References
-
- Lechan RM, Hollenberg A, Fekete C. Hypothalamic-pituitary-thyroid axis: organization, neural/endocrine control of TRH. In: Squire LR, ed. Encyclopedia of Neuroscience. Oxford, UK: Academic Press; 2009:75–87
-
- Reichlin S. TRH: historical aspects. Ann NY Acad Sci. 1989;553:1–6 - PubMed
-
- Boler J, Enzmann F, Folkers K, Bowers CY, Schally AV. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem Biophys Res Commun. 1969;37:705–710 - PubMed
-
- Burgus R, Dunn TF, Desiderio D, Guillemin R. [Molecular structure of the hypothalamic hypophysiotropic TRF factor of ovine origin: mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence]. C R Acad Sci Hebd Seances Acad Sci D. 1969;269:1870–1873 - PubMed
-
- Hashimoto K, Zanger K, Hollenberg AN, Cohen LE, Radovick S, Wondisford FE. cAMP response element-binding protein-binding protein mediates thyrotropin-releasing hormone signaling on thyrotropin subunit genes. J Biol Chem. 2000;275:33365–33372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

