Torin1-mediated TOR kinase inhibition reduces Wee1 levels and advances mitotic commitment in fission yeast and HeLa cells
- PMID: 24424027
- PMCID: PMC3953821
- DOI: 10.1242/jcs.146373
Torin1-mediated TOR kinase inhibition reduces Wee1 levels and advances mitotic commitment in fission yeast and HeLa cells
Abstract
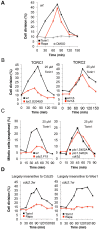
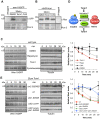
The target of rapamycin (TOR) kinase regulates cell growth and division. Rapamycin only inhibits a subset of TOR activities. Here we show that in contrast to the mild impact of rapamycin on cell division, blocking the catalytic site of TOR with the Torin1 inhibitor completely arrests growth without cell death in Schizosaccharomyces pombe. A mutation of the Tor2 glycine residue (G2040D) that lies adjacent to the key Torin-interacting tryptophan provides Torin1 resistance, confirming the specificity of Torin1 for TOR. Using this mutation, we show that Torin1 advanced mitotic onset before inducing growth arrest. In contrast to TOR inhibition with rapamycin, regulation by either Wee1 or Cdc25 was sufficient for this Torin1-induced advanced mitosis. Torin1 promoted a Polo and Cdr2 kinase-controlled drop in Wee1 levels. Experiments in human cell lines recapitulated these yeast observations: mammalian TOR (mTOR) was inhibited by Torin1, Wee1 levels declined and mitotic commitment was advanced in HeLa cells. Thus, the regulation of the mitotic inhibitor Wee1 by TOR signalling is a conserved mechanism that helps to couple cell cycle and growth controls.
Keywords: HeLa; S. pombe; TOR; Torin1; Wee1.
Figures






References
-
- Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y - DOI - PubMed
-
- Caspari T., Dahlen M., Kanter-Smoler G., Lindsay H. D., Hofmann K., Papadimitriou K., Sunnerhagen P., Carr A. M. (2000). Characterization of Schizosaccharomyces pombe Hus1: a PCNA-related protein that associates with Rad1 and Rad9. Mol. Cell. Biol. 20, 1254–1262 10.1128/MCB.20.4.1254-1262.2000 - DOI - PMC - PubMed
-
- Costello G., Rodgers L., Beach D. (1986). Fission yeast enters the stationary phase g0 state from either mitotic g1 or g2. Curr. Genet. 11, 119–125 10.1007/BF00378203 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

