The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings
- PMID: 24424103
- PMCID: PMC3982909
- DOI: 10.1016/j.clinbiochem.2014.01.002
The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings
Abstract
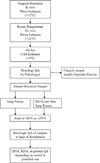
Well preserved frozen biospecimens are ideal for evaluating the genome, transcriptome, and proteome. While papers reviewing individual aspects of frozen biospecimens are available, we present a current overview of experimental data regarding procurement, storage, and quality assurance that can inform the handling of frozen biospecimens. Frozen biospecimen degradation can be influenced by factors independent of the collection methodology including tissue type, premortem agonal changes, and warm ischemia time during surgery. Rapid stabilization of tissues by snap freezing immediately can mitigate artifactually altered gene expression and, less appreciated, protein phosphorylation profiles. Collection protocols may be adjusted for specific tissue types as cellular ischemia tolerance varies widely. If data is not available for a particular tissue type, a practical goal is snap freezing within 20min. Tolerance for freeze-thaw events is also tissue type dependent. Tissue storage at -80°C can preserve DNA and protein for years but RNA can show degradation at 5years. For -80°C freezers, aliquots frozen in RNAlater or similar RNA stabilizing solutions are a consideration. It remains unresolved as to whether storage at -150°C provides significant advantages relative to that at -80°C. Histologic quality assurance of tissue biospecimens is typically performed at the time of surgery but should also be conducted on the aliquot to be distributed because of tissue heterogeneity. Biobanking protocols for blood and its components are highly dependent on intended use and multiple collection tube types may be needed. Additional quality assurance testing should be dictated by the anticipated downstream applications.
Keywords: Biobank; Biorepository; Biospecimen; Frozen; Procurement; Tissue.
Copyright © 2014 The Canadian Society of Clinical Chemists. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures
References
-
- World population prospects. The 2012 Revision by the United Nations Department of Economic and Social Affairs, Population Division. [Accessed October 21, 2013]; http://esa.un.org/wpp/Documentation/publications.htm.
-
- Vaught J, Rogers J, Carolin T, Compton C. Biobankonomics: Developing a Sustainable Business Model Approach for the Formation of a Human Tissue Biobank. J Natl Cancer Inst Monogr. 2011;42:24–31. - PubMed
-
- Naber SP. Continuing role of a frozen-tissue bank in molecular pathology. Diagn Mol Pathol. 1996;5:253–259. - PubMed
-
- Fairley JA, Gilmour K, Walsh K. Making the most of pathological specimens: molecular diagnosis in formalin-fixed, paraffin embedded tissue. Curr Drug Targets. 2012;13:1475–1487. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources