Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: a possible role in protection against oxidative DNA damage
- PMID: 24424315
- PMCID: PMC3907852
- DOI: 10.3390/ijms15011003
Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: a possible role in protection against oxidative DNA damage
Abstract
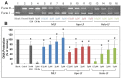
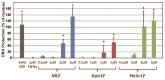
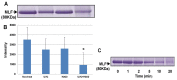
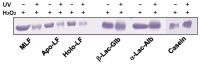
In this study, we examined the protective effect of lactoferrin against DNA damage induced by various hydroxyl radical generation systems. Lactoferrin (LF) was examined with regard to its potential role as a scavenger against radical oxygen species using bovine milk LF. Native LF, iron-saturated LF (holo-LF), and apolactoferrin (apo-LF) effectively suppressed strand breaks in plasmid DNA due to hydroxyl radicals produced by the Fenton reaction. In addition, both native LF and holo-LF clearly protected calf thymus DNA from fragmentation due to ultraviolet irradiation in the presence of H2O2. We also demonstrated a protective effect of all three LF molecules against 8-hydroxydeoxyguanosine (8-OHdG) formation in calf thymus DNA following ultraviolet (UV) irradiation with H2O2. Our results clearly indicate that native LF has reactive oxygen species-scavenging ability, independent of its nature as a masking component for transient metals. We also demonstrated that the protective effect of LF against oxidative DNA damage is due to degradation of LF itself, which is more susceptible to degradation than other bovine milk proteins.
Figures


References
-
- Schanbacher F.L., Goodman R.E., Talhouk R.S. Bovine mammary lactoferrin: Implications from messenger ribonucleic acid (mRNA) sequence and regulation contrary to other milk proteins. J. Dairy. Sci. 1993;76:3812–3831. - PubMed
-
- van der Strate B.W., Beljaars L., Molema G., Harmsen M.C., Meijer D.K. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. - PubMed
-
- Bennett R.M., Kokocinski T. Lactoferrin content of peripheral blood cells. Br. J. Haematol. 1978;39:509–521. - PubMed
-
- Caccavo D., Pellegrino N.M., Altamura M., Rigon A., Amati L., Amoroso A., Jirillo E. Antimicrobial and immunoregulatory functions of lactoferrin and its potential therapeutic application. J. Endotoxin Res. 2002;8:403–417. - PubMed
-
- Conneely O.M. Antiinflammatory activities of lactoferrin. J. Am. Coll. Nutr. 2001;20:389S–395S. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

