The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato
- PMID: 24424324
- PMCID: PMC3938611
- DOI: 10.1104/pp.113.233395
The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato
Abstract
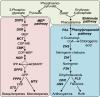
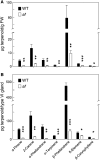
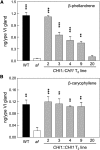
Flavonoids and terpenoids are derived from distinct metabolic pathways but nevertheless serve complementary roles in mediating plant interactions with the environment. Here, we show that glandular trichomes of the anthocyanin free (af) mutant of cultivated tomato (Solanum lycopersicum) fail to accumulate both flavonoids and terpenoids. This pleiotropic metabolic deficiency was associated with loss of resistance to native populations of coleopteran herbivores under field conditions. We demonstrate that Af encodes an isoform (SlCHI1) of the flavonoid biosynthetic enzyme chalcone isomerase (CHI), which catalyzes the conversion of naringenin chalcone to naringenin and is strictly required for flavonoid production in multiple tissues of tomato. Expression of the wild-type SlCHI1 gene from its native promoter complemented the anthocyanin deficiency in af. Unexpectedly, the SlCHI1 transgene also complemented the defect in terpenoid production in glandular trichomes. Our results establish a key role for SlCHI1 in flavonoid production in tomato and reveal a link between CHI1 and terpenoid production. Metabolic coordination of the flavonoid and terpenoid pathways may serve to optimize the function of trichome glands in dynamic environments.
Figures





References
-
- Aziz N, Paiva NL, May GD, Dixon RA. (2005) Transcriptome analysis of alfalfa glandular trichomes. Planta 221: 28–38 - PubMed
-
- Bednar RA, Hadcock JR. (1988) Purification and characterization of chalcone isomerase from soybeans. J Biol Chem 263: 9582–9588 - PubMed
-
- Bedon F, Bomal C, Caron S, Levasseur C, Boyle B, Mansfield SD, Schmidt A, Gershenzon J, Grima-Pettenati J, Séguin A, et al. (2010) Subgroup 4 R2R3-MYBs in conifer trees: gene family expansion and contribution to the isoprenoid- and flavonoid-oriented responses. J Exp Bot 61: 3847–3864 - PMC - PubMed
-
- Ben Zvi MM, Shklarman E, Masci T, Kalev H, Debener T, Shafir S, Ovadis M, Vainstein A. (2012) PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol 195: 335–345 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

