Targeting Müller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity
- PMID: 24425851
- PMCID: PMC3920823
- DOI: 10.1167/iovs.13-13755
Targeting Müller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity
Abstract
Purpose: To determine whether knockdown of Müller cell-derived VEGFA-splice variant, VEGF164, which is upregulated in the rat retinopathy of prematurity (ROP) model, safely inhibits intravitreal neovascularization (IVNV).
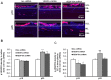
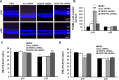
Methods: Short hairpin RNAs for VEGF164 (VEGF164.shRNAs) or luciferase.shRNA control were cloned into lentivectors with CD44 promoters that specifically target Müller cells. Knockdown efficiency, off-target effects, and specificity were tested in HEK reporter cell lines that expressed green fluorescent protein (GFP)-tagged VEGF164 or VEGF120 with flow cytometry or in rat Müller cells (rMC-1) by real-time PCR. In the rat oxygen-induced retinopathy (OIR) ROP model, pups received 1 μL subretinal lentivector-driven luciferase.shRNA, VEGFA.shRNA, or VEGF164.shRNA at postnatal day 8 (P8). Analyses at P18 and P25 included: IVNV and avascular retina (AVA); retinal and serum VEGF (ELISA); density of phosphorylated VEGFR2 (p-VEGFR2) in lectin-labeled retinal endothelial cells (ECs; immunohistochemistry); TUNEL staining and thickness of inner nuclear (INL) and outer nuclear layers (ONL) in retinal cryosections; and pup weight gain.
Results: In HEK reporter and in rMC-1 cells and in comparison to lucifferase.shRNA, VEGFA.shRNA reduced both VEGF120 and VEGF164, but VEGF164.shRNA only reduced VEGF164 and not VEGF120. Compared with luciferase.shRNA, VEGFA.shRNA and VEGF164.shRNA reduced retinal VEGF and IVNV without affecting AVA at P18 and P25. At P25, VEGF164.shRNA more effectively maintained IVNV inhibition than VEGFA.shRNA. VEGFA.shRNA and VEGF164.shRNA reduced pVEGFR2 in retinal ECs at P18, but VEGFA.shRNA increased it at P25. VEGFA.shRNA increased TUNEL+ cells at P18 and decreased ONL thickness at P18 and P25. VEGFA.shRNA and VEGF164.shRNA did not affect pup weight gain and serum VEGF.
Conclusions: Short hairpin RNA to Müller cell VEGF164 maintained long-term inhibition of IVNV and limited cell death compared with shRNA to VEGFA.
Keywords: Müller cells; intravitreal neovascularization; lentivector; short hairpin RNA; vascular endothelial growth factor.
Figures




Similar articles
-
Short hairpin RNA-mediated knockdown of VEGFA in Müller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity.Am J Pathol. 2013 Sep;183(3):964-74. doi: 10.1016/j.ajpath.2013.05.011. Am J Pathol. 2013. PMID: 23972394 Free PMC article.
-
Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity.Invest Ophthalmol Vis Sci. 2014 Feb 4;55(2):737-44. doi: 10.1167/iovs.13-13429. Invest Ophthalmol Vis Sci. 2014. PMID: 24425858 Free PMC article.
-
Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Müller cells maintains retinal function by triggering different signaling mechanisms.Sci Rep. 2018 Jan 31;8(1):2003. doi: 10.1038/s41598-018-20278-4. Sci Rep. 2018. PMID: 29386650 Free PMC article.
-
Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis).Trans Am Ophthalmol Soc. 2010 Dec;108:96-119. Trans Am Ophthalmol Soc. 2010. PMID: 21212851 Free PMC article. Review.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
-
RNA-Seq Provides Insights into VEGF-Induced Signaling in Human Retinal Microvascular Endothelial Cells: Implications in Retinopathy of Prematurity.Int J Mol Sci. 2022 Jul 1;23(13):7354. doi: 10.3390/ijms23137354. Int J Mol Sci. 2022. PMID: 35806359 Free PMC article.
-
Genomics in the neonatal nursery: Focus on ROP.Semin Perinatol. 2015 Dec;39(8):604-10. doi: 10.1053/j.semperi.2015.09.007. Epub 2015 Oct 23. Semin Perinatol. 2015. PMID: 26477493 Free PMC article. Review.
-
Effect of subretinal injection on retinal structure and function in a rat oxygen-induced retinopathy model.Mol Vis. 2017 Nov 29;23:832-843. eCollection 2017. Mol Vis. 2017. PMID: 29259390 Free PMC article.
-
Retinal Inhibition of CCR3 Induces Retinal Cell Death in a Murine Model of Choroidal Neovascularization.PLoS One. 2016 Jun 16;11(6):e0157748. doi: 10.1371/journal.pone.0157748. eCollection 2016. PLoS One. 2016. PMID: 27309355 Free PMC article.
-
Advances in diagnosis, clinical care, research, and treatment in retinopathy of prematurity.Eye Brain. 2016;8:27-29. doi: 10.2147/EB.S105319. Epub 2016 May 19. Eye Brain. 2016. PMID: 27795669 Free PMC article. No abstract available.
References
-
- Churchill AJ, Carter JG, Lovell HC, et al. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006; 15: 2955–2961 - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487 - PubMed
-
- Nicholson B, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010; 248: 915–930 - PubMed
-
- Cooke RWI, Drury JA, Mountford R, Clark D. Genetic Polymorphisms and Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2004; 45: 1712–1715 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

