Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity
- PMID: 24425858
- PMCID: PMC3915769
- DOI: 10.1167/iovs.13-13429
Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity
Abstract
Purpose: Targeted inhibition of Müller cell (MC)-produced VEGF or broad inhibition of VEGF with an intravitreal anti-VEGF antibody reduces intravitreal neovascularization in a rat model of retinopathy of prematurity (ROP). In this study, we compared the effects of these two approaches on retinal vascular development and capillary density in the inner and deep plexi in the rat ROP model.
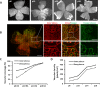
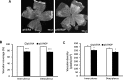
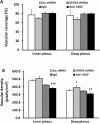
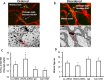
Methods: In the rat model of ROP, pups received 1 μL of (1) subretinal lentivector-driven short hairpin RNA (shRNA) to knockdown MC-VEGFA (VEGFA.shRNA) or control luciferase shRNA, or (2) intravitreal anti-VEGF antibody (anti-VEGF) or control isotype goat immunoglobulin G (IgG). Analyses of lectin-stained flat mounts at postnatal day 18 (p18) included: vascular/total retinal areas (retinal vascular coverage) and pixels of fluorescence/total retinal area (capillary density) of the inner and deep plexi determined with the Syncroscan microscope, and angles between cleavage planes of mitotic vascular figures labeled with anti-phosphohistone H3 and vessel length.
Results: Retinal vascular coverage and density increased in both plexi between p8 and p18 in room air (RA) pups. Compared with RA, p18 ROP pups had reduced vascular coverage and density of both plexi. Compared with respective controls, VEGFA.shRNA treatment significantly increased vascular density in the deep plexus, whereas anti-VEGF reduced vascular density in the inner and deep plexi. Vascular endothelial growth factor-A.shRNA caused more cleavage angles predicting vessel elongation and fewer mitotic figures, whereas anti-VEGF treatment led to patterns of pathologic angiogenesis.
Conclusions: Targeted treatment with lentivector-driven VEGFA.shRNA permitted physiologic vascularization of the vascular plexi and restored normal orientation of dividing vascular cells, suggesting that regulation of VEGF signaling by targeted treatment may be beneficial.
Keywords: VEGF; intrvitreal neovscularization; rat model of retinopathy of prematurity; vascular density; vascular extent.
Figures

References
-
- Chan-Ling T, Gock B, Stone J. Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1995; 36: 1215–1230 - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

