Semienzymatic cyclization of disulfide-rich peptides using Sortase A
- PMID: 24425873
- PMCID: PMC3945325
- DOI: 10.1074/jbc.M113.539262
Semienzymatic cyclization of disulfide-rich peptides using Sortase A
Abstract
Disulfide-rich cyclic peptides have generated great interest in the development of peptide-based therapeutics due to their exceptional stability toward chemical, enzymatic, or thermal attack. In particular, they have been used as scaffolds onto which bioactive epitopes can be grafted to take advantage of the favorable biophysical properties of disulfide-rich cyclic peptides. To date, the most commonly used method for the head-to-tail cyclization of peptides has been native chemical ligation. In recent years, however, enzyme-mediated cyclization has become a promising new technology due to its efficiency, safety, and cost-effectiveness. Sortase A (SrtA) is a bacterial enzyme with transpeptidase activity. It recognizes a C-terminal penta-amino acid motif, LPXTG, and cleaves the amide bond between Thr and Gly to form a thioacyl-linked intermediate. This intermediate undergoes nucleophilic attack by an N-terminal poly-Gly sequence to form an amide bond between the Thr and N-terminal Gly. Here, we demonstrate that sortase A can successfully be used to cyclize a variety of small disulfide-rich peptides, including the cyclotide kalata B1, α-conotoxin Vc1.1, and sunflower trypsin inhibitor 1. These peptides range in size from 14 to 29 amino acids and contain three, two, or one disulfide bond, respectively, within their head-to-tail cyclic backbones. Our findings provide proof of concept for the potential broad applicability of enzymatic cyclization of disulfide-rich peptides with therapeutic potential.
Keywords: Cyclic Peptides; Cyclotides; Disulfide; Enzymatic Cyclization; Kalata B1; NMR; Peptide Chemical Synthesis; Peptides; Sortase A; Toxins.
Figures

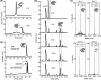
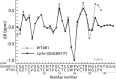



References
-
- Colgrave M. L., Craik D. J. (2004) Thermal, chemical, and enzymatic stability of the cyclotide kalata B1. The importance of the cyclic cystine knot. Biochemistry 43, 5965–5975 - PubMed
-
- Gran L. (1970) An oxytocic principle found in Oldenlandia affinis DC. An indigenous, Congolese drug “Kalata-Kalata” used to accelerate delivery. Medd. Nor. Farm. Selsk. 32, 173–180
-
- Clark R. J., Jensen J., Nevin S. T., Callaghan B. P., Adams D. J., Craik D. J. (2010) The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angew. Chem. Int. Ed. Engl. 49, 6545–6548 - PubMed
-
- Wong C. T., Rowlands D. K., Wong C. H., Lo T. W., Nguyen G. K., Li H. Y., Tam J. P. (2012) Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew. Chem. Int. Ed. Engl. 51, 5620–5624 - PubMed
-
- Cemazar M., Kwon S., Mahatmanto T., Ravipati A. S., Craik D. J. (2012) Discovery and applications of disulfide-rich cyclic peptides. Curr. Top. Med. Chem. 12, 1534–1545 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

