Hepatic gluconeogenesis is enhanced by phosphatidic acid which remains uninhibited by insulin in lipodystrophic Agpat2-/- mice
- PMID: 24425876
- PMCID: PMC3931038
- DOI: 10.1074/jbc.M113.530998
Hepatic gluconeogenesis is enhanced by phosphatidic acid which remains uninhibited by insulin in lipodystrophic Agpat2-/- mice
Abstract
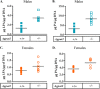
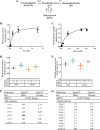
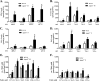
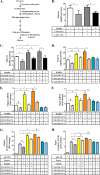
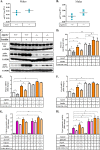
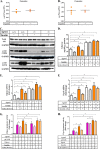
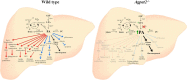
In this study we examined the role of phosphatidic acid (PA) in hepatic glucose production (HGP) and development of hepatic insulin resistance in mice that lack 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2). Liver lysophosphatidic acid and PA levels were increased ∼2- and ∼5-fold, respectively, in male Agpat2(-/-) mice compared with wild type mice. In the absence of AGPAT2, the liver can synthesize PAs by activating diacylglycerol kinase or phospholipase D, both of which were elevated in the livers of Agpat2(-/-) mice. We found that PAs C16:0/18:1 and C18:1/20:4 enhanced HGP in primary WT hepatocytes, an effect that was further enhanced in primary hepatocytes from Agpat2(-/-) mice. Lysophosphatidic acids C16:0 and C18:1 failed to increase HGP in primary hepatocytes. The activation of HGP was accompanied by an up-regulation of the key gluconeogenic enzymes glucose-6-phosphatase and phosphoenolpyruvate carboxykinase. This activation was suppressed by insulin in the WT primary hepatocytes but not in the Agpat2(-/-) primary hepatocytes. Thus, the lack of normal insulin signaling in Agpat2(-/-) livers allows unrestricted PA-induced gluconeogenesis significantly contributing to the development of hyperglycemia in these mice.
Keywords: Gluconeogenesis; Insulin Resistance; Lipids; Lipodystrophy; Phosphatidic Acid.
Figures


Similar articles
-
The Role of the AGPAT2 Gene in Adipose Tissue Biology and Congenital Generalized Lipodystrophy Pathophysiology.Int J Mol Sci. 2025 Jun 5;26(11):5416. doi: 10.3390/ijms26115416. Int J Mol Sci. 2025. PMID: 40508223 Free PMC article. Review.
-
Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2-/- lipodystrophic mice independent of hepatocyte leptin receptors.J Lipid Res. 2014 Feb;55(2):276-88. doi: 10.1194/jlr.M045799. Epub 2013 Nov 30. J Lipid Res. 2014. PMID: 24293639 Free PMC article.
-
Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice.J Biol Chem. 2011 Oct 28;286(43):37676-91. doi: 10.1074/jbc.M111.250449. Epub 2011 Aug 27. J Biol Chem. 2011. PMID: 21873652 Free PMC article.
-
Lysophosphatidic acid triggers inflammation in the liver and white adipose tissue in rat models of 1-acyl-sn-glycerol-3-phosphate acyltransferase 2 deficiency and overnutrition.Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2312666120. doi: 10.1073/pnas.2312666120. Epub 2023 Dec 21. Proc Natl Acad Sci U S A. 2023. PMID: 38127985 Free PMC article.
-
Genetic basis of congenital generalized lipodystrophy.Int J Obes Relat Metab Disord. 2004 Feb;28(2):336-9. doi: 10.1038/sj.ijo.0802487. Int J Obes Relat Metab Disord. 2004. PMID: 14557833 Review.
Cited by
-
The Role of the AGPAT2 Gene in Adipose Tissue Biology and Congenital Generalized Lipodystrophy Pathophysiology.Int J Mol Sci. 2025 Jun 5;26(11):5416. doi: 10.3390/ijms26115416. Int J Mol Sci. 2025. PMID: 40508223 Free PMC article. Review.
-
A Lipidomic Readout of Disease Progression in A Diet-Induced Mouse Model of Nonalcoholic Fatty Liver Disease.Trans Am Clin Climatol Assoc. 2015;126:271-88. Trans Am Clin Climatol Assoc. 2015. PMID: 26330688 Free PMC article.
-
Involvement of a battery of investigated genes in lipid droplet pathophysiology and associated comorbidities.Adipocyte. 2024 Dec;13(1):2403380. doi: 10.1080/21623945.2024.2403380. Epub 2024 Sep 27. Adipocyte. 2024. PMID: 39329369 Free PMC article. Review.
-
Congenital generalized lipodystrophies--new insights into metabolic dysfunction.Nat Rev Endocrinol. 2015 Sep;11(9):522-34. doi: 10.1038/nrendo.2015.123. Epub 2015 Aug 4. Nat Rev Endocrinol. 2015. PMID: 26239609 Free PMC article. Review.
-
Characterization of the Mouse and Human Monoacylglycerol O-Acyltransferase 1 (Mogat1) Promoter in Human Kidney Proximal Tubule and Rat Liver Cells.PLoS One. 2016 Sep 9;11(9):e0162504. doi: 10.1371/journal.pone.0162504. eCollection 2016. PLoS One. 2016. PMID: 27611931 Free PMC article.
References
-
- Agarwal A. K., Garg A. (2003) Congenital generalized lipodystrophy. Significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 14, 214–221 - PubMed
-
- Coleman R. A., Lee D. P. (2004) Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 43, 134–176 - PubMed
-
- Agarwal A. K. (2012) Lysophospholipid acyltransferases. 1-Acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr. Opin Lipidol. 23, 290–302 - PubMed
-
- Agarwal A. K., Arioglu E., De Almeida S., Akkoc N., Taylor S. I., Bowcock A. M., Barnes R. I., Garg A. (2002) AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 31, 21–23 - PubMed
-
- Vantyghem M. C., Balavoine A. S., Douillard C., Defrance F., Dieudonne L., Mouton F., Lemaire C., Bertrand-Escouflaire N., Bourdelle-Hego M. F., Devemy F., Evrard A., Gheerbrand D., Girardot C., Gumuche S., Hober C., Topolinski H., Lamblin B., Mycinski B., Ryndak A., Karrouz W., Duvivier E., Merlen E., Cortet C., Weill J., Lacroix D., Wemeau J. L. (2012) How to diagnose a lipodystrophy syndrome. Ann. Endocrinol. 73, 170–189 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

