Copper(II)-mediated thermolysis of alginates: a model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae
- PMID: 24427515
- PMCID: PMC3638285
- DOI: 10.1098/rsfs.2012.0046
Copper(II)-mediated thermolysis of alginates: a model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae
Abstract
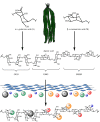
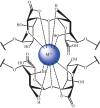
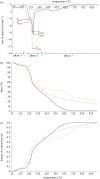
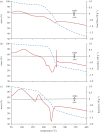
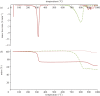
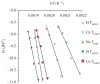
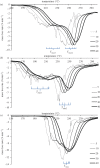
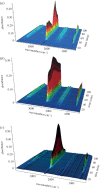
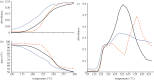
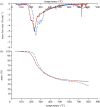
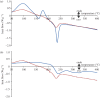
Thermochemical processing methods such as pyrolysis are of growing interest as a means of converting biomass into fuels and commodity chemicals in a sustainable manner. Macroalgae, or seaweed, represent a novel class of feedstock for pyrolysis that, owing to the nature of the environments in which they grow coupled with their biochemistry, naturally possess high metal contents. Although the impact of metals upon the pyrolysis of terrestrial biomass is well documented, their influence on the thermochemical conversion of marine-derived feeds is largely unknown. Furthermore, these effects are inherently difficult to study, owing to the heterogeneous character of natural seaweed samples. The work described in this paper uses copper(II) alginate, together with alginic acid and sodium alginate as model compounds for exploring the effects of metals upon macroalgae thermolysis. A thermogravimetric analysis-Fourier transform infrared spectroscopic study revealed that, unusually, Cu(2+) ions promote the onset of pyrolysis in the alginate polymer, with copper(II) alginate initiating rapid devolatilization at 143°C, 14°C lower than alginic acid and 61°C below the equivalent point for sodium alginate. Moreover, this effect was mirrored in a sample of wild Laminaria digitata that had been doped with Cu(2+) ions prior to pyrolysis, thus validating the use of alginates as model compounds with which to study the thermolysis of macroalgae. These observations indicate the varying impact of different metal species on thermochemical behaviour of seaweeds and offer an insight into the pyrolysis of brown macroalgae used in phytoremediation of metal-containing waste streams.
Keywords: alginate; biofuel; copper; pyrolysis; seaweed; thermochemistry.
Figures


References
-
- Giampietro M, Mayumi K. 2009. The biofuel delusion: the fallacy of large-scale agro-biofuel production. London, UK: Earthscan
-
- Inderwildi O, King D. 2009. Quo vadis biofuels? Energy Environ. Sci. 2, 343–346 10.1039/b822951c (doi:10.1039/b822951c) - DOI - DOI
-
- Bridgwater AV. 2006. Biomass for energy. J. Sci. Food. Agric. 86, 1755–1768 10.1002/jsfa.2605 (doi:10.1002/jsfa.2605) - DOI - DOI
-
- Goyal HB, Seal D, Saxena RC. 2008. Bio-fuels from thermochemical conversion of renewable resources: a review. Renew. Sust. Energy Rev. 12, 504–517 10.1016/j.rser.2006.07.014 (doi:10.1016/j.rser.2006.07.014) - DOI - DOI
-
- Mohan D, Pittman CU, Steele PH. 2006. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20, 848–889 10.1021/ef0502397 (doi:10.1021/ef0502397) - DOI - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
