Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses
- PMID: 24427764
- PMCID: PMC3890452
- DOI: 10.7774/cevr.2014.3.1.58
Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses
Abstract
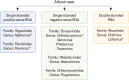
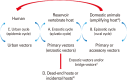
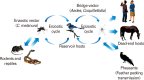
In this review, we mainly focus on zoonotic encephalitides caused by arthropod-borne viruses (arboviruses) of the families Flaviviridae (genus Flavivirus) and Togaviridae (genus Alphavirus) that are important in both humans and domestic animals. Specifically, we will focus on alphaviruses (Eastern equine encephalitis virus, Western equine encephalitis virus, Venezuelan equine encephalitis virus) and flaviviruses (Japanese encephalitis virus and West Nile virus). Most of these viruses were originally found in tropical regions such as Africa and South America or in some regions in Asia. However, they have dispersed widely and currently cause diseases around the world. Global warming, increasing urbanization and population size in tropical regions, faster transportation and rapid spread of arthropod vectors contribute in continuous spreading of arboviruses into new geographic areas causing reemerging or resurging diseases. Most of the reemerging arboviruses also have emerged as zoonotic disease agents and created major public health issues and disease epidemics.
Keywords: Alphaviruses; Arboviruses; Arthropod-borne viruses; Encephalitis; Equine Encephalomyelitis; Flaviviruses; Zoonoses.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




References
-
- JOINT WHO/FAO expert committee on zoonoses. World Health Organ Tech Rep Ser. 1959;58:1–84. - PubMed
-
- Soldan SS, Gonzalez-Scarano F. Emerging infectious diseases: the Bunyaviridae. J Neurovirol. 2005;11:412–423. - PubMed
-
- Elliott R. Bunyaviruses: general features. In: Mahy BW, van Regenmortel MH, editors. Encyclopedia of virology. London: Elsevier-Academic Press; 2008. pp. 390–399.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

