Excitation wavelength dependent O2 release from copper(II)-superoxide compounds: laser flash-photolysis experiments and theoretical studies
- PMID: 24428309
- PMCID: PMC3936478
- DOI: 10.1021/ja4115314
Excitation wavelength dependent O2 release from copper(II)-superoxide compounds: laser flash-photolysis experiments and theoretical studies
Abstract
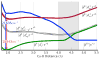
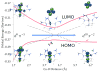
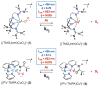
Irradiation of the copper(II)-superoxide synthetic complexes [(TMG3tren)Cu(II)(O2)](+) (1) and [(PV-TMPA)Cu(II)(O2)](+) (2) with visible light resulted in direct photogeneration of O2 gas at low temperature (from -40 °C to -70 °C for 1 and from -125 to -135 °C for 2) in 2-methyltetrahydrofuran (MeTHF) solvent. The yield of O2 release was wavelength dependent: λexc = 436 nm, ϕ = 0.29 (for 1), ϕ = 0.11 (for 2), and λexc = 683 nm, ϕ = 0.035 (for 1), ϕ = 0.078 (for 2), which was followed by fast O2-recombination with [(TMG3tren)Cu(I)](+) (3) and [(PV-TMPA)Cu(I)](+) (4). Enthalpic barriers for O2 rebinding to the copper(I) center (∼10 kJ mol(-1)) and for O2 dissociation from the superoxide compound 1 (45 kJ mol(-1)) were determined. TD-DFT studies, carried out for 1, support the experimental results confirming the dissociative character of the excited states formed upon blue- or red-light laser excitation.
Figures


Similar articles
-
Laser-Induced Dynamics of Peroxodicopper(II) Complexes Vary with the Ligand Architecture. One-Photon Two-Electron O2 Ejection and Formation of Mixed-Valent Cu(I)Cu(II)-Superoxide Intermediates.J Am Chem Soc. 2015 Dec 23;137(50):15865-74. doi: 10.1021/jacs.5b10177. Epub 2015 Dec 11. J Am Chem Soc. 2015. PMID: 26651492 Free PMC article.
-
The rate of O2 and CO binding to a copper complex, determined by a "flash-and-trap" technique, exceeds that for hemes.J Am Chem Soc. 2003 Oct 1;125(39):11866-71. doi: 10.1021/ja034911v. J Am Chem Soc. 2003. PMID: 14505408
-
Further insights into the spectroscopic properties, electronic structure, and kinetics of formation of the heme-peroxo-copper complex [(F8TPP)FeIII-(O2(2-)-CuII(TMPA)]+.Inorg Chem. 2007 May 14;46(10):3889-902. doi: 10.1021/ic061726k. Epub 2007 Apr 20. Inorg Chem. 2007. PMID: 17444630
-
Ultrafast excited-state dynamics of copper(I) complexes.Acc Chem Res. 2015 Mar 17;48(3):782-91. doi: 10.1021/ar500353h. Epub 2015 Feb 3. Acc Chem Res. 2015. PMID: 25646861 Review.
-
[CuO](+) and [CuOH](2+) complexes: intermediates in oxidation catalysis?Acc Chem Res. 2015 Jul 21;48(7):2126-31. doi: 10.1021/acs.accounts.5b00169. Epub 2015 Jun 15. Acc Chem Res. 2015. PMID: 26075312 Free PMC article. Review.
Cited by
-
Copper-Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity.Chem Rev. 2017 Feb 8;117(3):2059-2107. doi: 10.1021/acs.chemrev.6b00636. Epub 2017 Jan 19. Chem Rev. 2017. PMID: 28103018 Free PMC article. Review.
-
Benchmark Density Functional Theory Approach for the Calculation of Bond Dissociation Energies of the M-O2 Bond: A Key Step in Water Splitting Reactions.ACS Omega. 2022 Jun 9;7(24):20800-20808. doi: 10.1021/acsomega.2c01331. eCollection 2022 Jun 21. ACS Omega. 2022. PMID: 35935283 Free PMC article.
-
Light-Induced Reactivity Switch at O2-Activating Bioinspired Copper(I) Complexes.JACS Au. 2024 Apr 29;4(5):1966-1974. doi: 10.1021/jacsau.4c00184. eCollection 2024 May 27. JACS Au. 2024. PMID: 38818064 Free PMC article.
-
Laser-Induced Dynamics of Peroxodicopper(II) Complexes Vary with the Ligand Architecture. One-Photon Two-Electron O2 Ejection and Formation of Mixed-Valent Cu(I)Cu(II)-Superoxide Intermediates.J Am Chem Soc. 2015 Dec 23;137(50):15865-74. doi: 10.1021/jacs.5b10177. Epub 2015 Dec 11. J Am Chem Soc. 2015. PMID: 26651492 Free PMC article.
-
Peroxo and Superoxo Moieties Bound to Copper Ion: Electron-Transfer Equilibrium with a Small Reorganization Energy.J Am Chem Soc. 2016 Jun 8;138(22):7055-66. doi: 10.1021/jacs.6b02404. Epub 2016 May 26. J Am Chem Soc. 2016. PMID: 27228314 Free PMC article.
References
-
- Fukuzumi S, Karlin KD. Coord Chem Rev. 2013;257:187. - PMC - PubMed
- Himes RA, Karlin KD. Curr Opin Chem Biol. 2009;13:119. - PMC - PubMed
- Solomon EI, Ginsbach JW, Heppner DE, Kieber-Emmons MT, Kjaergaard CH, Smeets PJ, Tian L, Woertink JS. Faraday Discuss. 2011;148:11. - PMC - PubMed
- Allen SE, Walvoord RR, Padilla-Salinas R, Kozlowski MC. Chem, Rev. 2013;113:6234. - PMC - PubMed
-
- Maiti D, Lee DH, Gaoutchenova K, Wurtele C, Holthausen MC, Sarjeant AA, Sundermeyer J, Schindler S, Karlin KD. Angew Chem, Int Ed. 2008;47:82. - PubMed
-
- Comba P, Knoppe S, Martin B, Rajaraman G, Rolli C, Shapiro B, Stork T. Chemistry. 2008;14:344. - PubMed
- Decker A, Solomon EI. Curr Opin Chem Biol. 2005;9:152. - PubMed
- Yoshizawa K, Kihara N, Kamachi T, Shiota Y. Inorg Chem. 2006;45:3034. - PubMed
- Crespo A, Marti MA, Roitberg AE, Amzel LM, Estrin DA. J Am Chem Soc. 2006;128:12817. - PubMed
- Huber SM, Ertem MZ, Aquilante F, Gagliardi L, Tolman WB, Cramer CJ. Chemistry. 2009;15:4886. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

