Necrotizing meningoencephalitis in atypical dog breeds: a case series and literature review
- PMID: 24428322
- PMCID: PMC4895549
- DOI: 10.1111/jvim.12233
Necrotizing meningoencephalitis in atypical dog breeds: a case series and literature review
Abstract
Background: Canine necrotizing meningoencephalitis (NME) is a fatal, noninfectious inflammatory disease of unknown etiology. NME has been reported only in a small number of dog breeds, which has led to the presumption that it is a breed-restricted disorder.
Hypothesis/objectives: Our objective was to describe histopathologically confirmed NME in dog breeds in which the condition has not been reported previously and to provide preliminary evidence that NME affects a wider spectrum of dog breeds than previously reported.
Animals: Four dogs with NME.
Methods: Archives from 3 institutions and from 1 author's (BS) collection were reviewed to identify histopathologically confirmed cases of NME in breeds in which the disease has not been reported previously. Age, sex, breed, survival from onset of clinical signs, and histopathologic findings were evaluated.
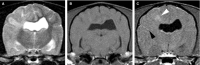
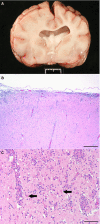
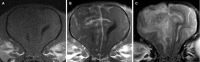
Results: Necrotizing meningoencephalitis was identified in 4 small dog breeds (Papillon, Shih Tzu, Coton de Tulear, and Brussels Griffon). Median age at clinical evaluation was 2.5 years. Histopathologic abnormalities included 2 or more of the following: lymphoplasmacytic or histiocytic meningoencephalitis or encephalitis, moderate-to-severe cerebrocortical necrosis, variable involvement of other anatomic locations within the brain (cerebellum, brainstem), and absence of detectable infectious agents.
Conclusions and clinical importance: Until now, NME has only been described in 5 small dog breeds. We document an additional 4 small breeds previously not shown to develop NME. Our cases further illustrate that NME is not a breed-restricted disorder and should be considered in the differential diagnosis for dogs with signalment and clinical signs consistent with inflammatory brain disease.
Keywords: Autoimmune; Dog; Inflammatory; Intracranial; Seizures.
Copyright © 2013 by the American College of Veterinary Internal Medicine.
Figures

References
-
- Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: A review and future perspectives. J Small Anim Pract 2010;51:138–149. - PubMed
-
- Barber RM, Schatzberg SJ, Corneveaux JJ, et al. Identification of risk loci for necrotizing meningoencephalitis in Pug dogs. J Hered 2011;102(Suppl 1):S40–S46. - PubMed
-
- Greer KA, Schatzberg SJ, Porter BF, et al. Heritability and transmission analysis of necrotizing meningoencephalitis in the Pug. Res Vet Sci 2009;86:438–442. - PubMed
-
- Cordy DR, Holliday TA. A necrotizing meningoencephalitis of Pug dogs. Vet Pathol 1989;26:191–194. - PubMed
-
- DeLahunta A, Glass E. Veterinary Neuroanatomy and Clinical Neurology, 3rd ed St. Louis, MO: Saunders Elsevier; 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

