Enhanced cell-specific ablation in zebrafish using a triple mutant of Escherichia coli nitroreductase
- PMID: 24428354
- PMCID: PMC3992008
- DOI: 10.1089/zeb.2013.0937
Enhanced cell-specific ablation in zebrafish using a triple mutant of Escherichia coli nitroreductase
Abstract
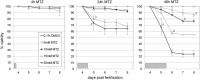
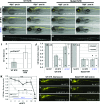
Transgenic expression of bacterial nitroreductase (NTR) facilitates chemically-inducible targeted cell ablation. In zebrafish, the NTR system enables studies of cell function and cellular regeneration. Metronidazole (MTZ) has become the most commonly used prodrug substrate for eliciting cell loss in NTR-expressing transgenic zebrafish due to the cell-specific nature of its cytotoxic derivatives. Unfortunately, MTZ treatments required for effective cell ablation border toxic effects, and, thus, likely incur undesirable nonspecific effects. Here, we tested whether a triple mutant variant of NTR, previously shown to display improved activity in bacterial assays, can solve this issue by promoting cell ablation in zebrafish using reduced prodrug treatment regimens. We generated several complementary transgenic zebrafish lines expressing either wild-type or mutant NTR (mutNTR) in specific neural cell types, and assayed prodrug-induced cell ablation kinetics using confocal time series imaging and plate reader-based quantification of fluorescent reporters expressed in targeted cell types. The results show that cell ablation can be achieved in mutNTR expressing transgenic lines with markedly shortened prodrug exposure times and/or at lower prodrug concentrations. The mutNTR variant characterized here can circumvent problematic nonspecific/toxic effects arising from low prodrug conversion efficiency, thus increasing the effectiveness and versatility of this selective cell ablation methodology.
Figures




References
-
- Zenno S, Koike H, Tanokura M, Saigo K. Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J Biochem 1996;120:736–744 - PubMed
-
- Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer 1995;31A:2362–2370 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

