Redox mediators in visible light photocatalysis: photocatalytic radical thiol-ene additions
- PMID: 24428433
- PMCID: PMC3985841
- DOI: 10.1021/jo500031g
Redox mediators in visible light photocatalysis: photocatalytic radical thiol-ene additions
Abstract
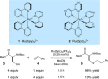
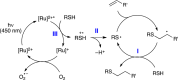
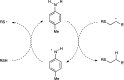
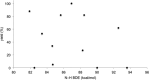
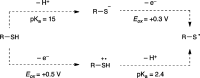
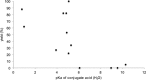
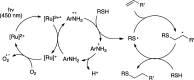
Synthetically useful radical thiol-ene reactions can be initiated by visible light irradiation in the presence of transition metal polypyridyl photocatalysts. The success of this method relies upon the use of p-toluidine as an essential additive. Using these conditions, high-yielding thiol-ene reactions of cysteine-containing biomolecules can be accomplished using biocompatibile wavelengths of visible light, under aqueous conditions, and with the thiol component as the limiting reagent. We present evidence that p-toluidine serves as a redox mediator that is capable of catalyzing the otherwise inefficient photooxidation of thiols to the key thiyl radical intermediate. Thus, we show that co-catalytic oxidants can be important in the design of synthetic reactions involving visible light photoredox catalysis.
Figures


References
-
- Dempsey J. L.; Winkler J. R.; Gray H. B. Chem. Rev. 2010, 110, 7024–7039. - PMC - PubMed
- Hartings M. R.; Kurnikov I. V.; Dunn A. R.; Winkler J. R.; Gray H. B.; Ratner M. A. Coord. Chem. Rev. 2010, 254, 248–253. - PMC - PubMed
- Barton J. K.; Olmon E. D.; Sontz P. A. Coord. Chem. Rev. 2011, 255, 619–634. - PMC - PubMed
- Gill M. R.; Thomas J. A. Chem. Soc. Rev. 2012, 41, 3179–3192. - PubMed
- Concepcion J. J.; Jurss J. W.; Brennaman A. O. T.; Iha N. Y. M.; Templeton J. L.; Meyer T. J. Acc. Chem. Res. 2009, 42, 1954–1965. - PubMed
- Alstrum-Acevedo J. H.; Brennaman M. K.; Meyer T. J. Inorg. Chem. 2005, 44, 6802–6827. - PubMed
-
-
For recent reviews, see:
- Zeitler K. Angew. Chem., Int. Ed. 2009, 48, 9785–9789. - PubMed
- Yoon T. P; Ischay M. A.; Du J. Nat. Chem. 2010, 2, 527–532. - PubMed
- Narayanam J. M. R.; Stephenson C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. - PubMed
- Teply F. Collect. Czech. Chem. Commun. 2011, 76, 859–917.
- Tucker J. W.; Stephenson C. R. J. J. Org. Chem. 2012, 77, 1617–1622. - PubMed
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 133, 5322–5363. - PMC - PubMed
-
-
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. - PubMed
- Du J.; Yoon T. P. J. Am. Chem. Soc. 2009, 131, 14604–14605. - PMC - PubMed
- Lu Z.; Shen M.; Yoon T. P. J. Am. Chem. Soc. 2011, 133, 1162–1164. - PMC - PubMed
- Hurtley A. E.; Cismesia M. A.; Ischay M. A.; Yoon T. P. Tetrahedron 2011, 67, 4442–4448. - PMC - PubMed
- Tyson E. L.; Farney E. P.; Yoon T. P. Org. Lett. 2012, 14, 1110–1113. - PMC - PubMed
-
- Lin S.; Ischay M. A.; Fry C. G.; Yoon T. P. J. Am. Chem. Soc. 2011, 133, 19350–19353. - PMC - PubMed
- Parrish J. D.; Ischay M. A.; Lu Z.; Guo S.; Peters N. R.; Yoon T. P. Org. Lett. 2012, 14, 1640–1643. - PMC - PubMed
- Ischay M. A.; Ament M. S.; Yoon T. P. Chem. Sci. 2012, 3, 2807–2811. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

