A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation
- PMID: 24428596
- PMCID: PMC3983127
- DOI: 10.1021/ar400181x
A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation
Abstract
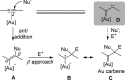
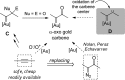
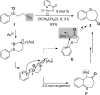
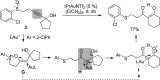
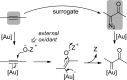
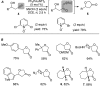
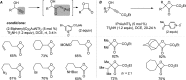
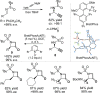
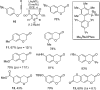
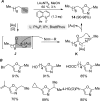
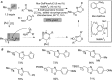
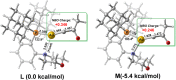
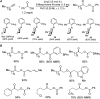
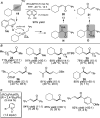
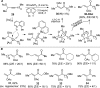
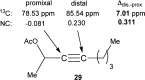
For the past dozen years, homogeneous gold catalysis has evolved from a little known topic in organic synthesis to a fully blown research field of significant importance to synthetic practitioners, due to its novel reactivities and reaction modes. Cationic gold(I) complexes are powerful soft Lewis acids that can activate alkynes and allenes toward efficient attack by nucleophiles, leading to the generation of alkenyl gold intermediates. Some of the most versatile aspects of gold catalysis involve the generation of gold carbene intermediates, which occurs through the approach of an electrophile to the distal end of the alkenyl gold moiety, and their diverse transformations thereafter. On the other hand, α-oxo metal carbene/carbenoids are highly versatile intermediates in organic synthesis and can undergo various synthetically challenging yet highly valuable transformations such as C-H insertion, ylide formation, and cyclopropanation reactions. Metal-catalyzed dediazotizations of diazo carbonyl compounds are the principle and most reliable strategy to access them. Unfortunately, the substrates contain a highly energetic diazo moiety and are potentially explosive. Moreover, chemists need to use energetic reagents to prepare them, putting further constrains on operational safety. In this Account, we show that the unique access to the gold carbene species in homogeneous gold catalysis offers an opportunity to generate α-oxo gold carbenes if both nucleophile and electrophile are oxygen. Hence, this approach would enable readily available and safer alkynes to replace hazardous α-diazo carbonyl compounds as precursors in the realm of gold carbene chemistry. For the past several years, we have demonstrated that alkynes can indeed effectively serve as precursors to versatile α-oxo gold carbenes. In our initial study, we showed that a tethered sulfoxide can be a suitable oxidant, which in some cases leads to the formation of α-oxo gold carbene intermediates. The intermolecular approach offers excellent synthetic flexibility because no tethering of the oxidant is required, and its reduced form is not tangled with the product. We were the first research group to develop this strategy, through the use of pyridine/quinolone N-oxides as the external oxidants. In this manner, we can effectively make a C-C triple bond a surrogate of an α-diazo carbonyl moiety in various gold catalyses. With terminal alkynes, we demonstrated that we can efficiently trap exclusively formed terminal carbene centers by internal nucleophiles en route to the formation of cyclic products, including strained oxetan-3-ones and azetidin-3-ones, and by external nucleophiles when a P,N-bidentate ligand is coordinated to gold. With internal alkynes, we generated synthetically useful regioselectivities in the generation of the α-oxo gold carbene moiety, which enables expedient formation of versatile enone products. Other research groups have also applied this strategy en route to versatile synthetic methods. The α-oxo gold carbenes appear to be more electrophilic than their Rh counterpart, which many chemists have focused on in a large array of excellent work on metal carbene chemistry. The ease of accessing the reactive gold carbenes opens up a vast area for developing new synthetic methods that would be distinctively different from the known Rh chemistry and promises to generate a new round of "gold rush".
Figures
References
-
- Hashmi A. S. K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. - PubMed
-
- Fürstner A.; Davies P. W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold π Acids. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. - PubMed
-
- Hashmi A. S. K.; Rudolph M. Gold Catalysis in Total Synthesis. Chem. Soc. Rev. 2008, 37, 1766–1775. - PubMed
-
- Rudolph M.; Hashmi A. S. K. Gold Catalysis in Total Synthesis-an Update. Chem. Soc. Rev. 2012, 41, 2448–2462. - PubMed
-
- Pauling L.The Nature of the Chemical Bond and the Structure of Molecules and Crystals; an Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: Ithaca, NY, 1960.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

