Safety and feasibility of oral immunotherapy to multiple allergens for food allergy
- PMID: 24428859
- PMCID: PMC3913318
- DOI: 10.1186/1710-1492-10-1
Safety and feasibility of oral immunotherapy to multiple allergens for food allergy
Erratum in
-
Erratum to: Safety and feasibility of oral immunotherapy to multiple allergens for food allergy.Allergy Asthma Clin Immunol. 2016 May 24;12:28. doi: 10.1186/s13223-016-0133-1. eCollection 2016. Allergy Asthma Clin Immunol. 2016. PMID: 27222658 Free PMC article.
Abstract
Background: Thirty percent of children with food allergy are allergic to more than one food. Previous studies on oral immunotherapy (OIT) for food allergy have focused on the administration of a single allergen at the time. This study aimed at evaluating the safety of a modified OIT protocol using multiple foods at one time.
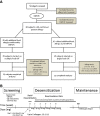
Methods: Participants underwent double-blind placebo-controlled food challenges (DBPCFC) up to a cumulative dose of 182 mg of food protein to peanut followed by other nuts, sesame, dairy or egg. Those meeting inclusion criteria for peanut only were started on single-allergen OIT while those with additional allergies had up to 5 foods included in their OIT mix. Reactions during dose escalations and home dosing were recorded in a symptom diary.
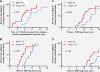
Results: Forty participants met inclusion criteria on peanut DBPCFC. Of these, 15 were mono-allergic to peanut and 25 had additional food allergies. Rates of reaction per dose did not differ significantly between the two groups (median of 3.3% and 3.7% in multi and single OIT group, respectively; p = .31). In both groups, most reactions were mild but two severe reactions requiring epinephrine occurred in each group. Dose escalations progressed similarly in both groups although, per protocol design, those on multiple food took longer to reach equivalent doses per food (median +4 mo.; p < .0001).
Conclusions: Preliminary data show oral immunotherapy using multiple food allergens simultaneously to be feasible and relatively safe when performed in a hospital setting with trained personnel. Additional, larger, randomized studies are required to continue to test safety and efficacy of multi-OIT.
Trial registration: Clinicaltrial.gov NCT01490177.
Figures


References
-
- Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rance F. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857–871. doi: 10.1111/j.1398-9995.2007.01421.x. - DOI - PubMed
-
- Hompes S, Kohli A, Nemat K, Scherer K, Lange L, Rueff F, Rietschel E, Reese T, Szepfalusi Z, Schwerk N. et al.Provoking allergens and treatment of anaphylaxis in children and adolescents–data from the anaphylaxis registry of German-speaking countries. Pediatr Allergy Immunol. 2011;22(6):568–574. doi: 10.1111/j.1399-3038.2011.01154.x. - DOI - PubMed
-
- Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, Henning AK, Stablein D, Sampson HA, Sicherer SH. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130(1):e25–e32. doi: 10.1542/peds.2011-1762. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

