Extracellular nucleotide catabolism by the Group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood
- PMID: 24429288
- PMCID: PMC3937624
- DOI: 10.1074/jbc.M113.545632
Extracellular nucleotide catabolism by the Group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood
Abstract
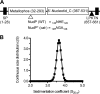
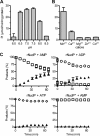
Streptococcus agalactiae (Group B Streptococcus) is a commensal of the human intestine and vagina of adult women but is the leading cause of invasive infection in neonates. This Gram-positive bacterium displays a set of virulence-associated surface proteins involved in the interaction with the host, such as adhesion to host cells, invasion of tissues, or subversion of the immune system. In this study, we characterized a cell wall-localized protein as an ecto-5'-nucleoside diphosphate phosphohydrolase (NudP) involved in the degradation of extracellular nucleotides which are central mediators of the immune response. Biochemical characterization of recombinant NudP revealed a Mn(2+)-dependent ecto-5'-nucleotidase activity on ribo- and deoxyribonucleoside 5'-mono- and 5'-diphosphates with a substrate specificity different from that of known orthologous enzymes. Deletion of the gene coding the housekeeping enzyme sortase A led to the release of NudP into the culture supernatant, confirming that this enzyme is anchored to the cell wall by its non-canonical LPXTN motif. The NudP ecto-5'-nucleotidase activity is reminiscent of the reactions performed by the mammalian ectonucleotidases CD39 and CD73 involved in regulating the extracellular level of ATP and adenosine. We further demonstrated that the absence of NudP activity decreases bacterial survival in mouse blood, a process dependent on extracellular adenosine. In vivo assays in animal models of infection showed that NudP activity is critical for virulence. These results demonstrate that Group B Streptococcus expresses a specific ecto-5'-nucleotidase necessary for its pathogenicity and highlight the diversity of reactions performed by this enzyme family. These results suggest that bacterial pathogens have developed specialized strategies to subvert the mammalian immune response controlled by the extracellular nucleotide signaling pathways.
Keywords: Adenosine; Bacterial Pathogenesis; Enzyme Catalysis; Enzymes; Host-Pathogen Interactions; Metalloenzymes; Nucleoside Nucleotide Metabolism; Streptococcus.
Figures


References
-
- Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., Ravichandran K. S. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 - PMC - PubMed
-
- Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

