Molecular mechanisms underlying synergistic adhesion of sickle red blood cells by hypoxia and low nitric oxide bioavailability
- PMID: 24429338
- PMCID: PMC3962164
- DOI: 10.1182/blood-2013-06-510180
Molecular mechanisms underlying synergistic adhesion of sickle red blood cells by hypoxia and low nitric oxide bioavailability
Abstract
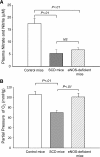
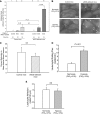
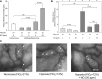
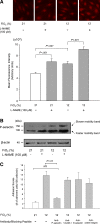
The molecular mechanisms by which nitric oxide (NO) bioavailability modulates the clinical expression of sickle cell disease (SCD) remain elusive. We investigated the effect of hypoxia and NO bioavailability on sickle red blood cell (sRBC) adhesion using mice deficient for endothelial NO synthase (eNOS) because their NO metabolite levels are similar to those of SCD mice but without hypoxemia. Whereas sRBC adhesion to endothelial cells in eNOS-deficient mice was synergistically upregulated at the onset of hypoxia, leukocyte adhesion was unaffected. Restoring NO metabolite levels to physiological levels markedly reduced sRBC adhesion to levels seen under normoxia. These results indicate that sRBC adherence to endothelial cells increases in response to hypoxia prior to leukocyte adherence, and that low NO bioavailability synergistically upregulates sRBC adhesion under hypoxia. Although multiple adhesion molecules mediate sRBC adhesion, we found a central role for P-selectin in sRBC adhesion. Hypoxia and low NO bioavailability upregulated P-selectin expression in endothelial cells in an additive manner through p38 kinase pathways. These results demonstrate novel cellular and signaling mechanisms that regulate sRBC adhesion under hypoxia and low NO bioavailability. Importantly, these findings point us toward new molecular targets to inhibit cell adhesion in SCD.
Figures

Comment in
-
No NO means yes to sickle red cell adhesion.Blood. 2014 Mar 20;123(12):1780-2. doi: 10.1182/blood-2014-02-551218. Blood. 2014. PMID: 24652963 No abstract available.
References
-
- Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. - PubMed
-
- Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J Mol Biol. 1985;183(4):591–610. - PubMed
-
- Ataga KI, Smith WR, De Castro LM, et al. ICA-17043-05 Investigators. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991–3997. - PubMed
-
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol. 2002;9(2):101–106. - PubMed
-
- Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22(6):515–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

