Nod-like receptor X-1 is required for rhinovirus-induced barrier dysfunction in airway epithelial cells
- PMID: 24429360
- PMCID: PMC3993547
- DOI: 10.1128/JVI.03039-13
Nod-like receptor X-1 is required for rhinovirus-induced barrier dysfunction in airway epithelial cells
Abstract
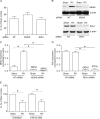
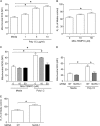
Barrier dysfunction of airway epithelium may increase the risk for acquiring secondary infections or allergen sensitization. Both rhinovirus (RV) and polyinosinic-polycytidilic acid [poly(I·C)], a double-stranded RNA (dsRNA) mimetic, cause airway epithelial barrier dysfunction, which is reactive oxygen species (ROS) dependent, implying that dsRNA generated during RV replication is sufficient for disrupting barrier function. We also demonstrated that RV or poly(I·C)-stimulated NADPH oxidase 1 (NOX-1) partially accounts for RV-induced ROS generation. In this study, we identified a dsRNA receptor(s) contributing to RV-induced maximal ROS generation and thus barrier disruption. We demonstrate that genetic silencing of the newly discovered dsRNA receptor Nod-like receptor X-1 (NLRX-1), but not other previously described dsRNA receptors, abrogated RV-induced ROS generation and reduction of transepithelial resistance (R(T)) in polarized airway epithelial cells. In addition, both RV and poly(I·C) stimulated mitochondrial ROS, the generation of which was dependent on NLRX-1. Treatment with Mito-Tempo, an antioxidant targeted to mitochondria, abolished RV-induced mitochondrial ROS generation, reduction in R(T), and bacterial transmigration. Furthermore, RV infection increased NLRX-1 localization to the mitochondria. Additionally, NLRX-1 interacts with RV RNA and poly(I·C) in polarized airway epithelial cells. Finally, we show that NLRX-1 is also required for RV-stimulated NOX-1 expression. These findings suggest a novel mechanism by which RV stimulates generation of ROS, which is required for disruption of airway epithelial barrier function.
Importance: Rhinovirus (RV), a virus responsible for a majority of common colds, disrupts the barrier function of the airway epithelium by increasing reactive oxygen species (ROS). Poly(I·C), a double-stranded RNA (dsRNA) mimetic, also causes ROS-dependent barrier disruption, implying that the dsRNA intermediate generated during RV replication is sufficient for this process. Here, we demonstrate that both RV RNA and poly(I·C) interact with NLRX-1 (a newly discovered dsRNA receptor) and stimulate mitochondrial ROS. We show for the first time that NLRX-1 is primarily expressed in the cytoplasm and at the apical surface rather than in the mitochondria and that NLRX-1 translocates to mitochondria following RV infection. Together, our results suggest a novel mechanism for RV-induced barrier disruption involving NLRX-1 and mitochondrial ROS. Although ROS is necessary for optimal viral clearance, if not neutralized efficiently, it may increase susceptibility to secondary infections and alter innate immune responses to subsequently inhaled pathogens, allergens, and other environmental factors.
Figures








Similar articles
-
Rhinovirus-Induced Modulation of Epithelial Phenotype: Role in Asthma.Viruses. 2020 Nov 19;12(11):1328. doi: 10.3390/v12111328. Viruses. 2020. PMID: 33227953 Free PMC article. Review.
-
Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1.J Virol. 2011 Jul;85(13):6795-808. doi: 10.1128/JVI.02074-10. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507984 Free PMC article.
-
FOXO3a regulates rhinovirus-induced innate immune responses in airway epithelial cells.Sci Rep. 2019 Dec 3;9(1):18180. doi: 10.1038/s41598-019-54567-3. Sci Rep. 2019. PMID: 31796819 Free PMC article.
-
Allergic environment enhances airway epithelial pro-inflammatory responses to rhinovirus infection.Clin Sci (Lond). 2017 Mar 1;131(6):499-509. doi: 10.1042/CS20160939. Epub 2017 Jan 23. Clin Sci (Lond). 2017. PMID: 28115681
-
Rhinovirus-Induced Airway Disease: A Model to Understand the Antiviral and Th2 Epithelial Immune Dysregulation in Childhood Asthma.J Investig Med. 2015 Aug;63(6):792-5. doi: 10.1097/JIM.0000000000000209. J Investig Med. 2015. PMID: 26057561 Free PMC article. Review.
Cited by
-
PGC-1α mediates a metabolic host defense response in human airway epithelium during rhinovirus infections.Nat Commun. 2021 Jun 16;12(1):3669. doi: 10.1038/s41467-021-23925-z. Nat Commun. 2021. PMID: 34135327 Free PMC article.
-
Male Mice Lacking NLRX1 Are Partially Protected From High-Fat Diet-Induced Hyperglycemia.J Endocr Soc. 2018 Feb 21;2(4):336-347. doi: 10.1210/js.2017-00360. eCollection 2018 Apr 1. J Endocr Soc. 2018. PMID: 29577109 Free PMC article.
-
Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine.J Innate Immun. 2018;10(5-6):487-501. doi: 10.1159/000487057. Epub 2018 Feb 13. J Innate Immun. 2018. PMID: 29439264 Free PMC article. Review.
-
Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure.J Gen Virol. 2016 Apr;97(4):825-838. doi: 10.1099/jgv.0.000401. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26763980 Free PMC article. Review.
-
Rhinovirus-Induced Modulation of Epithelial Phenotype: Role in Asthma.Viruses. 2020 Nov 19;12(11):1328. doi: 10.3390/v12111328. Viruses. 2020. PMID: 33227953 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

