A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC inhibitor, combined with erlotinib in patients with advanced aerodigestive tract tumors
- PMID: 24429877
- PMCID: PMC4051132
- DOI: 10.1158/1078-0432.CCR-13-2235
A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC inhibitor, combined with erlotinib in patients with advanced aerodigestive tract tumors
Abstract
Purpose: Panobinostat, a histone deacetylase (HDAC) inhibitor, enhances antiproliferative activity in non-small cell lung cancer (NSCLC) cell lines when combined with erlotinib. We evaluated this combination in patients with advanced NSCLC and head and neck cancer.
Experimental design: Eligible patients were enrolled in a 3+3 dose-escalation design to determine the maximum tolerated dose (MTD) of twice weekly panobinostat plus daily erlotinib at four planned dose levels (DL). Pharmacokinetics, blood, fat pad biopsies (FPB) for histone acetylation, and paired pre and posttherapy tumor biopsies for checkpoint kinase 1 (CHK1) expression were assessed.
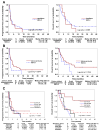
Results: Of 42 enrolled patients, 33 were evaluable for efficacy. Dose-limiting toxicities were prolonged-QTc and nausea at DL3. Adverse events included fatigue and nausea (grades 1-3), and rash and anorexia (grades 1-2). Disease control rates were 54% for NSCLC (n = 26) and 43% for head and neck cancer (n = 7). Of 7 patients with NSCLC with EGF receptor (EGFR) mutations, 3 had partial response, 3 had stable disease, and 1 progressed. For EGFR-mutant versus EGFR wild-type patients, progression-free survival (PFS) was 4.7 versus 1.9 months (P = 0.43) and overall survival was 41 (estimated) versus 5.2 months (P = 0.39). Erlotinib pharmacokinetics was not significantly affected. Correlative studies confirmed panobinostat's pharmacodynamic effect in blood, FPB, and tumor samples. Low CHK1 expression levels correlated with PFS (P = 0.006) and response (P = 0.02).
Conclusions: We determined MTD at 30 mg (panobinostat) and 100 mg (erlotinib). Further studies are needed to further explore the benefits of HDAC inhibitors in patients with EGFR-mutant NSCLC, investigate FPB as a potential surrogate source for biomarker investigations, and validate CHK1's predictive role.
©2014 AACR.
Conflict of interest statement
Employment or leadership position: N/A
Consultant or Advisory Role: N/A
Stock Ownership: N/A
Honoraria: Alberto Chiappori, MD (from Genentech)
Research Funding: N/A
Expert Testimony: N/A
Other Remuneration: N/A
Figures



References
-
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. - PubMed
-
- Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14:1497–511. - PubMed
-
- Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184:1–16. - PubMed
-
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. - PubMed
-
- Kim SC, SR, Chen Y, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

