Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication
- PMID: 24430183
- PMCID: PMC3904605
- DOI: 10.1172/JCI69519
Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication
Abstract
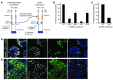
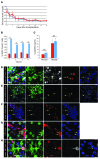
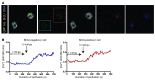
Children with focal hyperinsulinism of infancy display a dramatic, non-neoplastic clonal expansion of β cells that have undergone mitotic recombination, resulting in paternal disomy of part of chromosome 11. This disomic region contains imprinted genes, including the gene encoding the cell cycle inhibitor p57Kip2 (CDKN1C), which is silenced as a consequence of the recombination event. We hypothesized that targeting p57Kip2 could stimulate adult human β cell replication. Indeed, when we suppressed CDKN1C expression in human islets obtained from deceased adult organ donors and transplanted them into hyperglycemic, immunodeficient mice, β cell replication increased more than 3-fold. The newly replicated cells retained properties of mature β cells, including the expression of β cell markers such as insulin, PDX1, and NKX6.1. Importantly, these newly replicated cells demonstrated normal glucose-induced calcium influx, further indicating β cell functionality. These findings provide a molecular explanation for the massive β cell replication that occurs in children with focal hyperinsulinism. These data also provided evidence that β cells from older humans, in which baseline replication is negligible, can be coaxed to re-enter and complete the cell cycle while maintaining mature β cell properties. Thus, controlled manipulation of this pathway holds promise for the expansion of β cells in patients with type 2 diabetes.
Figures
References
-
- Aynsley-Green A. Nesidioblastosis of the pancreas in infancy. Dev Med Child Neurol. 1981;23(3):372–379. - PubMed
-
- Rahier J, Falt K, Muntefering H, Becker K, Gepts W, Falkmer S. The basic structural lesion of persistent neonatal hypoglycaemia with hyperinsulinism: deficiency of pancreatic D cells or hyperactivity of B cells? Diabetologia. 1984;26(4):282–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

