Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring
- PMID: 24430439
- PMCID: PMC5860829
- DOI: 10.2337/db13-1614
Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring
Abstract
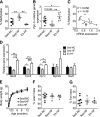
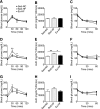
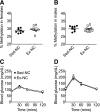
Abnormal conditions during early development adversely affect later health. We investigated whether maternal exercise could protect offspring from adverse effects of a maternal high-fat diet (HFD) with a focus on the metabolic outcomes and epigenetic regulation of the metabolic master regulator, peroxisome proliferator-activated receptor γ coactivator-1α (Pgc-1α). Female C57BL/6 mice were exposed to normal chow, an HFD, or an HFD with voluntary wheel exercise for 6 weeks before and throughout pregnancy. Methylation of the Pgc-1α promoter at CpG site -260 and expression of Pgc-1α mRNA were assessed in skeletal muscle from neonatal and 12-month-old offspring, and glucose and insulin tolerance tests were performed in the female offspring at 6, 9, and 12 months. Hypermethylation of the Pgc-1α promoter caused by a maternal HFD was detected at birth and was maintained until 12 months of age with a trend of reduced expression of Pgc-1α mRNA (P = 0.065) and its target genes. Maternal exercise prevented maternal HFD-induced Pgc-1α hypermethylation and enhanced Pgc-1α and its target gene expression, concurrent with amelioration of age-associated metabolic dysfunction at 9 months of age in the offspring. Therefore, maternal exercise is a powerful lifestyle intervention for preventing maternal HFD-induced epigenetic and metabolic dysregulation in the offspring.
Figures

Comment in
-
Comment on Laker et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014;63:1605-1611.Diabetes. 2014 May;63(5):e5. doi: 10.2337/db14-0086. Diabetes. 2014. PMID: 24757207 No abstract available.
-
Response to comment on Laker et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014;63:1605-1611.Diabetes. 2014 May;63(5):e6-7. doi: 10.2337/db14-0135. Diabetes. 2014. PMID: 24757208 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

