Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor
- PMID: 24430725
- PMCID: PMC3927118
- DOI: 10.1007/s40262-013-0126-x
Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor
Abstract
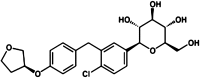
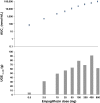
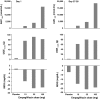
Empagliflozin is an orally active, potent and selective inhibitor of sodium glucose co-transporter 2 (SGLT2), currently in clinical development to improve glycaemic control in adults with type 2 diabetes mellitus (T2DM). SGLT2 inhibitors, including empagliflozin, are the first pharmacological class of antidiabetes agents to target the kidney in order to remove excess glucose from the body and, thus, offer new options for T2DM management. SGLT2 inhibitors exert their effects independently of insulin. Following single and multiple oral doses (0.5-800 mg), empagliflozin was rapidly absorbed and reached peak plasma concentrations after approximately 1.33-3.0 h, before showing a biphasic decline. The mean terminal half-life ranged from 5.6 to 13.1 h in single rising-dose studies, and from 10.3 to 18.8 h in multiple-dose studies. Following multiple oral doses, increases in exposure were dose-proportional and trough concentrations remained constant after day 6, indicating a steady state had been reached. Oral clearance at steady state was similar to corresponding single-dose values, suggesting linear pharmacokinetics with respect to time. No clinically relevant alterations in pharmacokinetics were observed in mild to severe hepatic impairment, or in mild to severe renal impairment and end-stage renal disease. Clinical studies did not reveal any relevant drug-drug interactions with several other drugs commonly prescribed to patients with T2DM, including warfarin. Urinary glucose excretion (UGE) rates were higher with empagliflozin versus placebo and increased with dose, but no relevant impact on 24-h urine volume was observed. Increased UGE resulted in proportional reductions in fasting plasma glucose and mean daily glucose concentrations.
Figures
References
-
- Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. - DOI - PubMed
-
- Toubro S, Cefalu WT, Xie J, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes [abstract no. 762] Diabetologia. 2012;55:S313.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

