SLC25A23 augments mitochondrial Ca²⁺ uptake, interacts with MCU, and induces oxidative stress-mediated cell death
- PMID: 24430870
- PMCID: PMC3952861
- DOI: 10.1091/mbc.E13-08-0502
SLC25A23 augments mitochondrial Ca²⁺ uptake, interacts with MCU, and induces oxidative stress-mediated cell death
Abstract
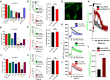
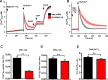
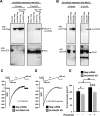
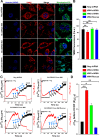
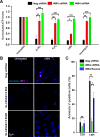
Emerging findings suggest that two lineages of mitochondrial Ca(2+) uptake participate during active and resting states: 1) the major eukaryotic membrane potential-dependent mitochondrial Ca(2+) uniporter and 2) the evolutionarily conserved exchangers and solute carriers, which are also involved in ion transport. Although the influx of Ca(2+) across the inner mitochondrial membrane maintains metabolic functions and cell death signal transduction, the mechanisms that regulate mitochondrial Ca(2+) accumulation are unclear. Solute carriers--solute carrier 25A23 (SLC25A23), SLC25A24, and SLC25A25--represent a family of EF-hand-containing mitochondrial proteins that transport Mg-ATP/Pi across the inner membrane. RNA interference-mediated knockdown of SLC25A23 but not SLC25A24 and SLC25A25 decreases mitochondrial Ca(2+) uptake and reduces cytosolic Ca(2+) clearance after histamine stimulation. Ectopic expression of SLC25A23 EF-hand-domain mutants exhibits a dominant-negative phenotype of reduced mitochondrial Ca(2+) uptake. In addition, SLC25A23 interacts with mitochondrial Ca(2+) uniporter (MCU; CCDC109A) and MICU1 (CBARA1) while also increasing IMCU. In addition, SLC25A23 knockdown lowers basal mROS accumulation, attenuates oxidant-induced ATP decline, and reduces cell death. Further, reconstitution with short hairpin RNA-insensitive SLC25A23 cDNA restores mitochondrial Ca(2+) uptake and superoxide production. These findings indicate that SLC25A23 plays an important role in mitochondrial matrix Ca(2+) influx.
Figures


References
-
- Amigo I, Traba J, Gonzalez-Barroso MM, Rueda CB, Fernandez M, Rial E, Sanchez A, Satrustegui J, Del Arco A. Glucagon regulation of oxidative phosphorylation requires an increase in matrix adenine nucleotide content through Ca2+ activation of the mitochondrial ATP-Mg/Pi carrier SCaMC-3. J Biol Chem. 2013;288:7791–7802. - PMC - PubMed
-
- Aprille JR. Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role. FASEB J. 1988;2:2547–2556. - PubMed
-
- Bassi MT, Manzoni M, Bresciani R, Pizzo MT, Della Monica A, Barlati S, Monti E, Borsani G. Cellular expression and alternative splicing of SLC25A23, a member of the mitochondrial Ca2+-dependent solute carrier gene family. Gene. 2005;345:173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

