Critical roles for multiple formins during cardiac myofibril development and repair
- PMID: 24430873
- PMCID: PMC3952851
- DOI: 10.1091/mbc.E13-08-0443
Critical roles for multiple formins during cardiac myofibril development and repair
Abstract
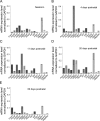
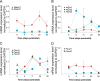
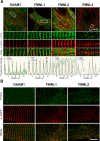
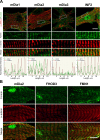
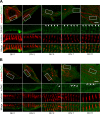
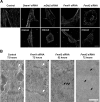
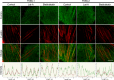
Cardiac and skeletal muscle function depends on the proper formation of myofibrils, which are tandem arrays of highly organized actomyosin contractile units called sarcomeres. How the architecture of these colossal molecular assemblages is established during development and maintained over the lifetime of an animal is poorly understood. We investigate the potential roles in myofibril formation and repair of formin proteins, which are encoded by 15 different genes in mammals. Using quantitative real-time PCR analysis, we find that 13 formins are differentially expressed in mouse hearts during postnatal development. Seven formins immunolocalize to sarcomeres in diverse patterns, suggesting that they have a variety of functional roles. Using RNA interference silencing, we find that the formins mDia2, DAAM1, FMNL1, and FMNL2 are required nonredundantly for myofibrillogenesis. Knockdown phenotypes include global loss of myofibril organization and defective sarcomeric ultrastructure. Finally, our analysis reveals an unanticipated requirement specifically for FMNL1 and FMNL2 in the repair of damaged myofibrils. Together our data reveal an unexpectedly large number of formins, with diverse localization patterns and nonredundant roles, functioning in myofibril development and maintenance, and provide the first evidence of actin assembly factors being required to repair myofibrils.
Figures


Similar articles
-
Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules.Mol Biol Cell. 2011 Dec;22(23):4575-87. doi: 10.1091/mbc.E11-07-0616. Epub 2011 Oct 12. Mol Biol Cell. 2011. PMID: 21998204 Free PMC article.
-
The actin-organizing formin protein Fhod3 is required for postnatal development and functional maintenance of the adult heart in mice.J Biol Chem. 2018 Jan 5;293(1):148-162. doi: 10.1074/jbc.M117.813931. Epub 2017 Nov 20. J Biol Chem. 2018. PMID: 29158260 Free PMC article.
-
DAAM1 and DAAM2 are co-required for myocardial maturation and sarcomere assembly.Dev Biol. 2015 Dec 1;408(1):126-39. doi: 10.1016/j.ydbio.2015.10.003. Epub 2015 Oct 23. Dev Biol. 2015. PMID: 26526197 Free PMC article.
-
Formins as effector proteins of Rho GTPases.Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914801 Free PMC article. Review.
-
Reforming the Barrier: The Role of Formins in Wound Repair.Cells. 2022 Sep 6;11(18):2779. doi: 10.3390/cells11182779. Cells. 2022. PMID: 36139355 Free PMC article. Review.
Cited by
-
The actin polymerization factor Diaphanous and the actin severing protein Flightless I collaborate to regulate sarcomere size.Dev Biol. 2021 Jan 1;469:12-25. doi: 10.1016/j.ydbio.2020.09.014. Epub 2020 Sep 25. Dev Biol. 2021. PMID: 32980309 Free PMC article.
-
The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles.Int J Mol Sci. 2022 May 10;23(10):5306. doi: 10.3390/ijms23105306. Int J Mol Sci. 2022. PMID: 35628117 Free PMC article. Review.
-
Integration of Cardiac Actin Mutants Causing Hypertrophic (p.A295S) and Dilated Cardiomyopathy (p.R312H and p.E361G) into Cellular Structures.Antioxidants (Basel). 2021 Jul 5;10(7):1082. doi: 10.3390/antiox10071082. Antioxidants (Basel). 2021. PMID: 34356314 Free PMC article.
-
Gene expression profiling reveals potential prognostic biomarkers associated with the progression of heart failure.Genome Med. 2015 Mar 14;7(1):26. doi: 10.1186/s13073-015-0149-z. eCollection 2015. Genome Med. 2015. PMID: 25984239 Free PMC article.
-
Ordering of myosin II filaments driven by mechanical forces: experiments and theory.Philos Trans R Soc Lond B Biol Sci. 2018 May 26;373(1747):20170114. doi: 10.1098/rstb.2017.0114. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29632266 Free PMC article. Review.
References
-
- Arber S, Hunter JJ, Ross J, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

