Complement inhibitors for age-related macular degeneration
- PMID: 24431152
- PMCID: PMC6885067
- DOI: 10.1002/14651858.CD009300.pub2
Complement inhibitors for age-related macular degeneration
Update in
-
Complement inhibitors for age-related macular degeneration.Cochrane Database Syst Rev. 2023 Jun 14;6(6):CD009300. doi: 10.1002/14651858.CD009300.pub3. Cochrane Database Syst Rev. 2023. PMID: 37314061 Free PMC article. Review.
Abstract
Background: Given the relatively high prevalence of age-related macular degeneration (AMD) and the increased incidence of AMD as populations age, the results of trials of novel treatments are awaited with much anticipation. The complement cascade describes a series of proteolytic reactions occurring throughout the body that generate proteins with a variety of roles including the initiation and promotion of immune reactions against foreign materials or micro-organisms. The complement cascade is normally tightly regulated, but much evidence implicates complement overactivity in AMD and so it is a logical therapeutic target in the treatment of AMD.
Objectives: To assess the effects and safety of complement inhibitors in the prevention or treatment of advanced AMD.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 11), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2013), EMBASE (January 1980 to November 2013), Allied and Complementary Medicine Database (AMED) (January 1985 to November 2013), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to November 2013), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), Web of Science Conference Proceedings Citation Index - Science (CPCI-S) (January 1990 to November 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 21 November 2013. We also performed handsearching of proceedings, from 2012 onwards, of meetings and conferences of specific professional organisations.
Selection criteria: We planned to include randomised controlled trials (RCTs) with parallel treatment groups which investigated either the prevention or treatment of advanced AMD by inhibition of the complement cascade.
Data collection and analysis: Two authors (MW and GMcK) independently evaluated all the titles and abstracts resulting from the searches. We contacted companies running clinical trials which had not yet reported results to request information. Since no trials met our inclusion criteria, we undertook no assessment of quality or meta-analysis.
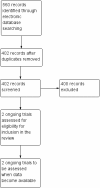
Main results: We identified and screened 317 references but there were no published RCTs that met the inclusion criteria. We identified two ongoing studies: one phase I study and one phase II study.
Authors' conclusions: There is insufficient information at present to generate evidence-based recommendations on the potential safety and efficacy of complement inhibitors for prevention or treatment of AMD. However we anticipate the results of ongoing trials.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
Similar articles
-
Aflibercept for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2016 Feb 8;2(2):CD011346. doi: 10.1002/14651858.CD011346.pub2. Cochrane Database Syst Rev. 2016. PMID: 26857947 Free PMC article.
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2014 Aug 29;8(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. Cochrane Database Syst Rev. 2014. PMID: 25170575 Free PMC article.
-
Surgery for cataracts in people with age-related macular degeneration.Cochrane Database Syst Rev. 2017 Feb 16;2(2):CD006757. doi: 10.1002/14651858.CD006757.pub4. Cochrane Database Syst Rev. 2017. PMID: 28206671 Free PMC article.
-
Statins for age-related macular degeneration.Cochrane Database Syst Rev. 2016 Aug 4;2016(8):CD006927. doi: 10.1002/14651858.CD006927.pub5. Cochrane Database Syst Rev. 2016. PMID: 27490232 Free PMC article.
-
Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration.Cochrane Database Syst Rev. 2012 Nov 14;11:CD000254. doi: 10.1002/14651858.CD000254.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jul 31;7:CD000254. doi: 10.1002/14651858.CD000254.pub4. PMID: 23152201 Updated.
Cited by
-
What do Cochrane systematic reviews say about interventions for age-related macular degeneration?Sao Paulo Med J. 2019 Nov-Dec;137(6):530-542. doi: 10.1590/1516-3180.2019.010317092019. Sao Paulo Med J. 2019. PMID: 32159640 Free PMC article. Review.
-
Interventions for Age-Related Macular Degeneration: Are Practice Guidelines Based on Systematic Reviews?Ophthalmology. 2016 Apr;123(4):884-97. doi: 10.1016/j.ophtha.2015.12.004. Epub 2016 Jan 22. Ophthalmology. 2016. PMID: 26804762 Free PMC article.
-
Anti-complement component 5 antibody targeting MG4 domain inhibits choroidal neovascularization.Oncotarget. 2017 Jul 11;8(28):45506-45516. doi: 10.18632/oncotarget.17221. Oncotarget. 2017. PMID: 28477014 Free PMC article.
-
Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age-related macular degeneration: Complement activation in AMD.Immun Ageing. 2016 Feb 16;13:4. doi: 10.1186/s12979-016-0060-5. eCollection 2016. Immun Ageing. 2016. PMID: 26884800 Free PMC article.
-
Molecular Features of Classic Retinal Drugs, Retinal Therapeutic Targets and Emerging Treatments.Pharmaceutics. 2021 Jul 20;13(7):1102. doi: 10.3390/pharmaceutics13071102. Pharmaceutics. 2021. PMID: 34371793 Free PMC article. Review.
References
References to ongoing studies
-
- NCT00935883. Eculizumab for the treatment of non‐exudative age‐related macular degeneration: an exploratory study to evaluate the effects of C5 inhibition on drusen and geographic atrophy. clinicaltrials.gov/show/NCT00935883 (accessed 28 May 2013).
-
- NCT00950638. A phase I study of ARC1905 (anti‐C5 aptamer) in subjects with dry age‐related macular degeneration. clinicaltrials.gov/show/Nct00950638 (accessed 28 May 2013).
Additional references
-
- Anonymous. Deal watch: Alcon licenses complement pathway inhibitor for macular degeneration. Nature Reviews Drug Discovery 2009;8:922. - PubMed
-
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology 2001;119(10):1417‐36. - PMC - PubMed
-
- Augood CA, Vingerling JR, Jong PT, Chakravarthy U, Seland J, Soubrane G, et al. Prevalence of age‐related maculopathy in older Europeans: the European Eye Study (EUREYE). Archives of Ophthalmology 2006;124(4):529‐35. - PubMed
-
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser‐induced choroidal neovascularization. Journal of Immunology 2005;174(1):491‐7. - PubMed
-
- Büttner‐Mainik A, Parsons J, Jérôme H, Hartmann A, Lamer S, Schaaf A, et al. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnology Journal 2011;9(3):373‐83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous