Heterotropic modulation of selectin affinity by allosteric antibodies affects leukocyte rolling
- PMID: 24431230
- PMCID: PMC3921101
- DOI: 10.4049/jimmunol.1302147
Heterotropic modulation of selectin affinity by allosteric antibodies affects leukocyte rolling
Abstract
Selectins are a family of adhesion receptors designed for efficient leukocyte tethering to the endothelium under shear. As a key property to resist premature bond disruption, selectin adhesiveness is enhanced by tensile forces that promote the conversion of a bent into an extended conformation of the N-terminal lectin and epidermal growth factor-like domains. Conformation-specific Abs have been invaluable in deciphering the activation mechanism of integrins, but similar reagents are not available for selectins. In this study, we show that the anti-human L-selectin mAbs DREG-55 and LAM1-5 but not DREG-56, DREG-200, or LAM1-1 heterotropically modulate adhesion presumably by stabilizing the extended receptor conformation. Force-free affinity assays, flow chamber, and microkinetic studies reveal a ligand-specific modulation of L-selectin affinity by DREG-55 mAb, resulting in a dramatic decrease of rolling velocity under flow. Furthermore, secondary tethering of polymorphonuclear cells was blocked by DREG-200 but significantly boosted by DREG-55 mAb. The results emphasize the need for a new classification for selectin Abs and introduce the new concept of heterotropic modulation of receptor function.
Figures


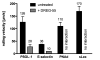


Similar articles
-
Targeting Neutrophil Adhesive Events to Address Vaso-Occlusive Crisis in Sickle Cell Patients.Front Immunol. 2021 Apr 28;12:663886. doi: 10.3389/fimmu.2021.663886. eCollection 2021. Front Immunol. 2021. PMID: 33995392 Free PMC article. Review.
-
Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force.Nat Immunol. 2006 Aug;7(8):883-9. doi: 10.1038/ni1366. Epub 2006 Jul 16. Nat Immunol. 2006. PMID: 16845394 Free PMC article.
-
Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins.Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3172-7. doi: 10.1073/pnas.94.7.3172. Proc Natl Acad Sci U S A. 1997. PMID: 9096365 Free PMC article.
-
Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin.J Immunol. 1997 Jan 1;158(1):367-75. J Immunol. 1997. PMID: 8977212
-
PSGL-1 function in immunity and steady state homeostasis.Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review.
Cited by
-
Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow.Blood. 2017 Nov 9;130(19):2101-2110. doi: 10.1182/blood-2017-05-783027. Epub 2017 Aug 15. Blood. 2017. PMID: 28811304 Free PMC article. Clinical Trial.
-
Stabilization of the Hinge Region of Human E-selectin Enhances Binding Affinity to Ligands Under Force.Cell Mol Bioeng. 2021 Feb 4;14(1):65-74. doi: 10.1007/s12195-021-00666-z. eCollection 2021 Feb. Cell Mol Bioeng. 2021. PMID: 33633813 Free PMC article.
-
Targeting Neutrophil Adhesive Events to Address Vaso-Occlusive Crisis in Sickle Cell Patients.Front Immunol. 2021 Apr 28;12:663886. doi: 10.3389/fimmu.2021.663886. eCollection 2021. Front Immunol. 2021. PMID: 33995392 Free PMC article. Review.
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. - PubMed
-
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr. Opin. Cell Biol. 2002;14:581–586. - PubMed
-
- Tu L, Murphy PG, Li X, Tedder TF. L-selectin ligands expressed by human leukocytes are HECA-452 antibody-defined carbohydrate epitopes preferentially displayed by P-selectin glycoprotein ligand-1. J. Immunol. Baltim. Md 1950. 1999;163:5070–5078. - PubMed
-
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

